SparQ™2 Cumate Switch System
SparQ™2 is adding some of the key advantages over the previous generation and possesses several important elements for researchers to conduct gene modulation projects.
- P2A linker provides higher consistency for GFP expression
- Insert gene size can now be larger than QM812B-1
- Robustness - increase expression up to 40-fold
- Adjustable - tune expression levels by titrating the amount of cumate
- Reversible - turn expression on, off, then on again
- Simple - the all-in-one format allows co-expression of CymR and your gene-of-interest
- Powerful - suitable for in vivo applications
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| QM820B-1 | Plasmid; pCDH-CuO-MCS-P2A-GFP-EF1a-CymR-T2A-Puro | 10 µg | $1753 |
|
||||
| QM821B-1 | Plasmid; pCDH-CuO-MCS-P2A-RFP-EF1-CymR-T2A-Puro | 10 µg | $1753 |
|
||||
| QM822B-1 | Plasmid; pCDH-CuO-MCS-P2A-GFP-EF1-CymR-T2A-Blast | 10 µg | $1753 |
|
||||
| QM823B-1 | Plasmid; pCDH-CuO-MCS-P2A-RFP-EF1-CymR-T2A-Blast | 10 µg | $1753 |
|
||||
| QM824B-1 | Plasmid; pCDH-CuO-MCS-P2A-GFP-EF1-CymR-T2A-Neo | 10 µg | $1753 |
|
||||
| QM825B-1 | Plasmid; pCDH-CuO-MCS-P2A-RFP-EF1-CymR-T2A-Neo | 10 µg | $1753 |
|
||||
| QM826B-1 | Plasmid; pCDH-CuO-RFP-P2A-GFP-EF1-CymR-T2A-Puro | 10 µg | $1753 |
|
||||
| QM828B-1 | Plasmid; pCDH-CuO-FLuc-P2A-GFP-EF1-CymR-T2A-Puro | 10 µg | $1753 |
|
||||
Overview
With SBI’s SparQ™2 Cumate Switch System, you can get inducible gene expression in mammalian cells with a range of cloning and expression lentivectors. It simplifies your SparQ™ projects by delivering your gene-of-interest in the same vector that has CymR built-in (vector shown below). The vector drives your gene-of-interest and fluorescent markers (GFP, or RFP) with the inducible cumate switch promoter, and constitutively co-expresses CymR and a resistance marker (puromycin, blasticidin, or neomycin) from an EF1α promoter.
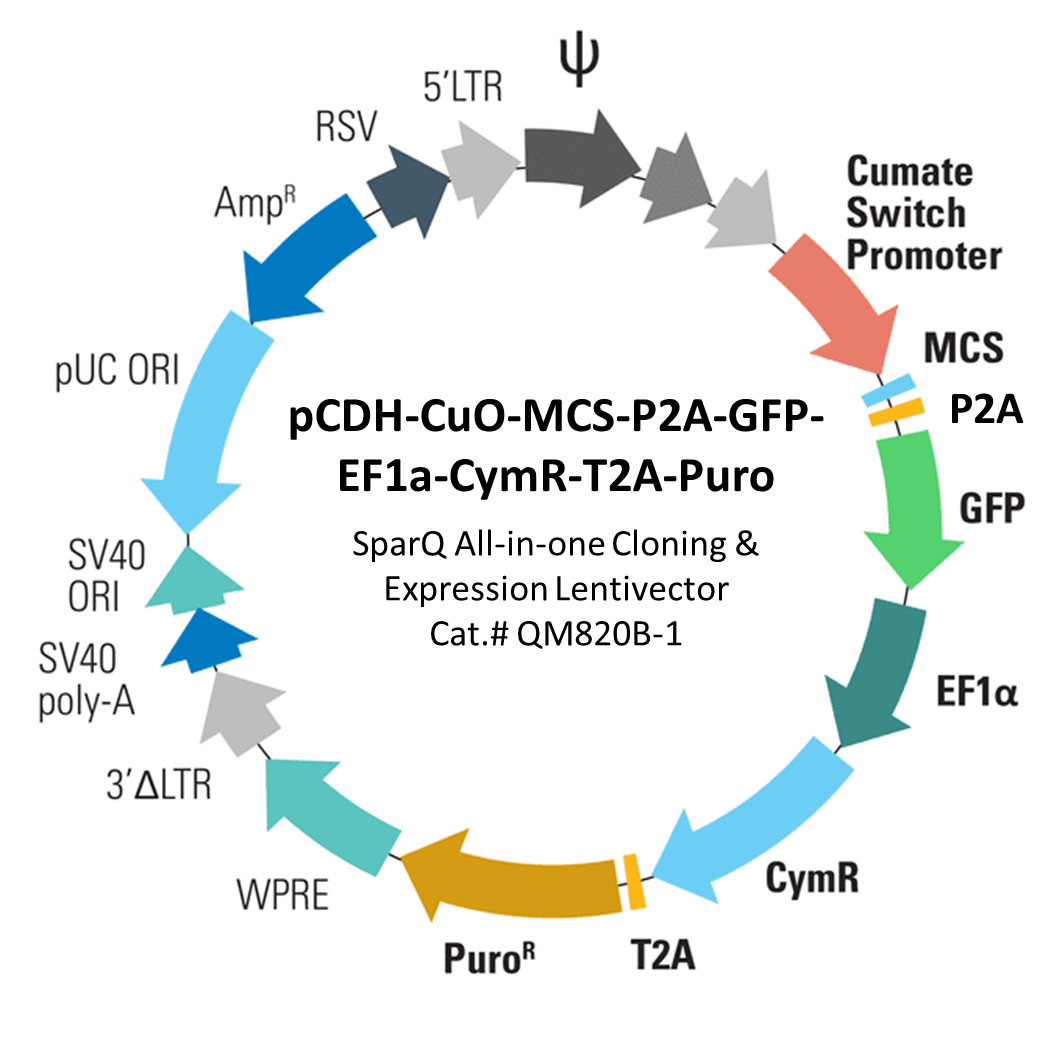
SBI’s SparQ™2 Cumate Switch System gives researchers the confidence when working with gene regulation in mammalian cells.
- Robustness - increase expression up to 40-fold
- Adjustable - tune expression levels by titrating the amount of cumate
- Reversible - turn expression on, off, then on again
- Simple - the all-in-one format allows co-expression of CymR and your gene-of-interest
- Powerful - suitable for in vivo applications
- Various marker selections (SparQ™2) - GFP or RFP in combination of puromycin, blasticidin, and neomycin
Overall, SparQ™ system is an excellent choice for achieving highest controlled levels of gene expression. It has negligible background expression in the absence of cumate and is a great approach to study cellular perturbation.
References
How It Works
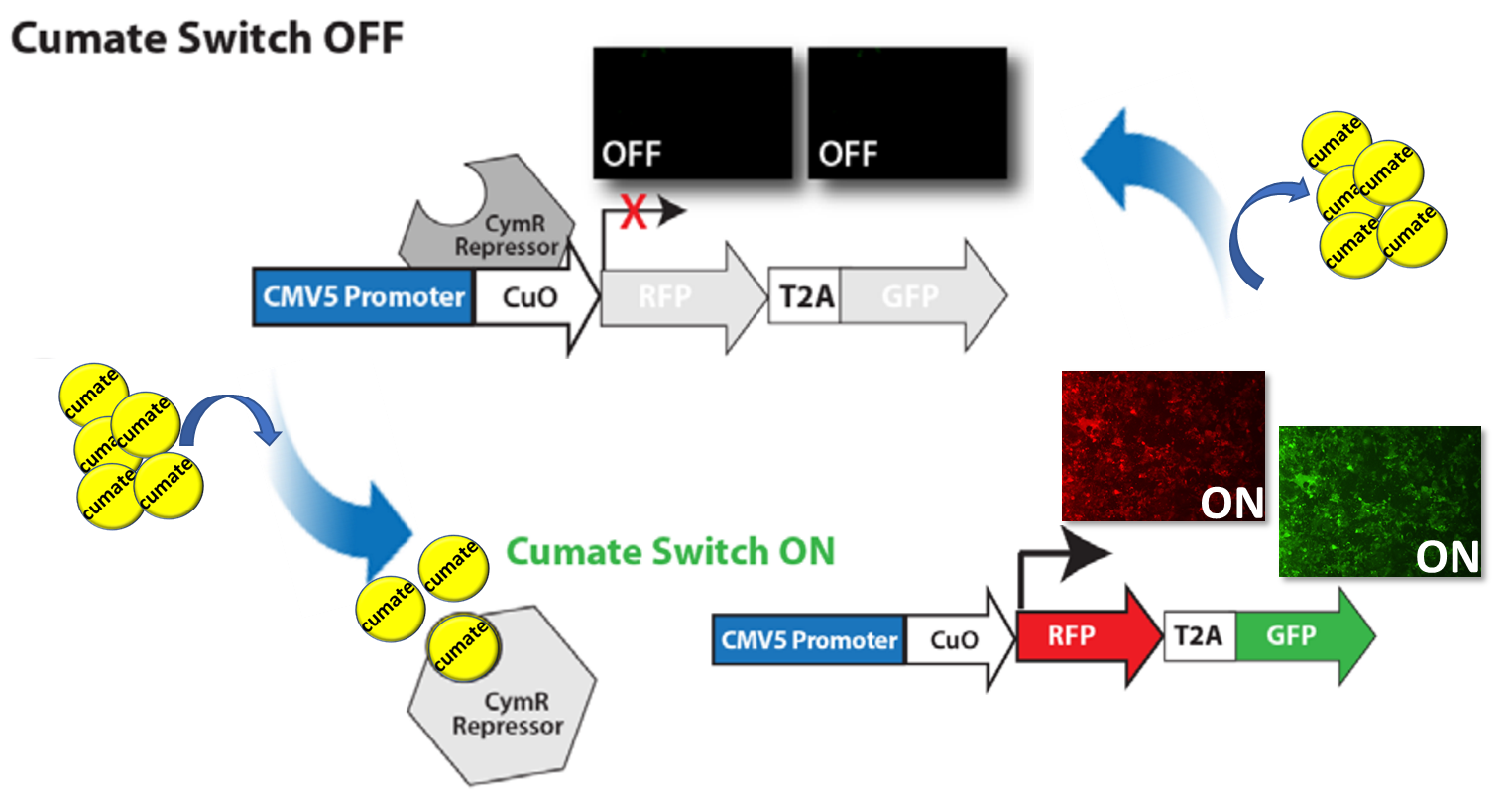
The regulatory mechanisms of the bacterial operons cmt and cym have been engineered to regulate gene expression in mammalian cells. In the repressor configuration, regulation is mediated by strong binding of the CymR repressor to the Cumate operator site (CuO), which is downstream of the CMV5 promoter. Addition of cumate (depicted as yellow circle in the graphic scheme below), a non-toxic small molecule inducer, relieves the repression and drives gene activation; in this case, fluorescent proteins (RFP and GFP). Subsequent removal of cumate from the growth media reverts the activation process, rendering gene switches to off mode.
SBI’s SparQ Cumate Switch System can be generalized as follows.
- CymR, a repressor that binds to cumate operator sequences in the absence of cumate
- MCS, to clone-in your gene-of-interest
- Cumate inducible promoter with cumate operator sequences (CuO) upstream of the MCS, to inducibly control gene expression
- Variety of selection markers, to monitor the induction or select stable cell line
- CymR has a high binding affinity for cumate and, as more cumate is added, fewer CymR molecules bind to the CuO sequences in the promoter resulting in increased expression
- Exhibiting much lower background expression than similar systems
Figure 1. Cumate Switch System
Supporting Data
Tight expression control, low background, and reversible with the SparQ™2 Cumate Switch System
Figure 2. Gene expression with the SparQ™2 Cumate Switch On/Off System
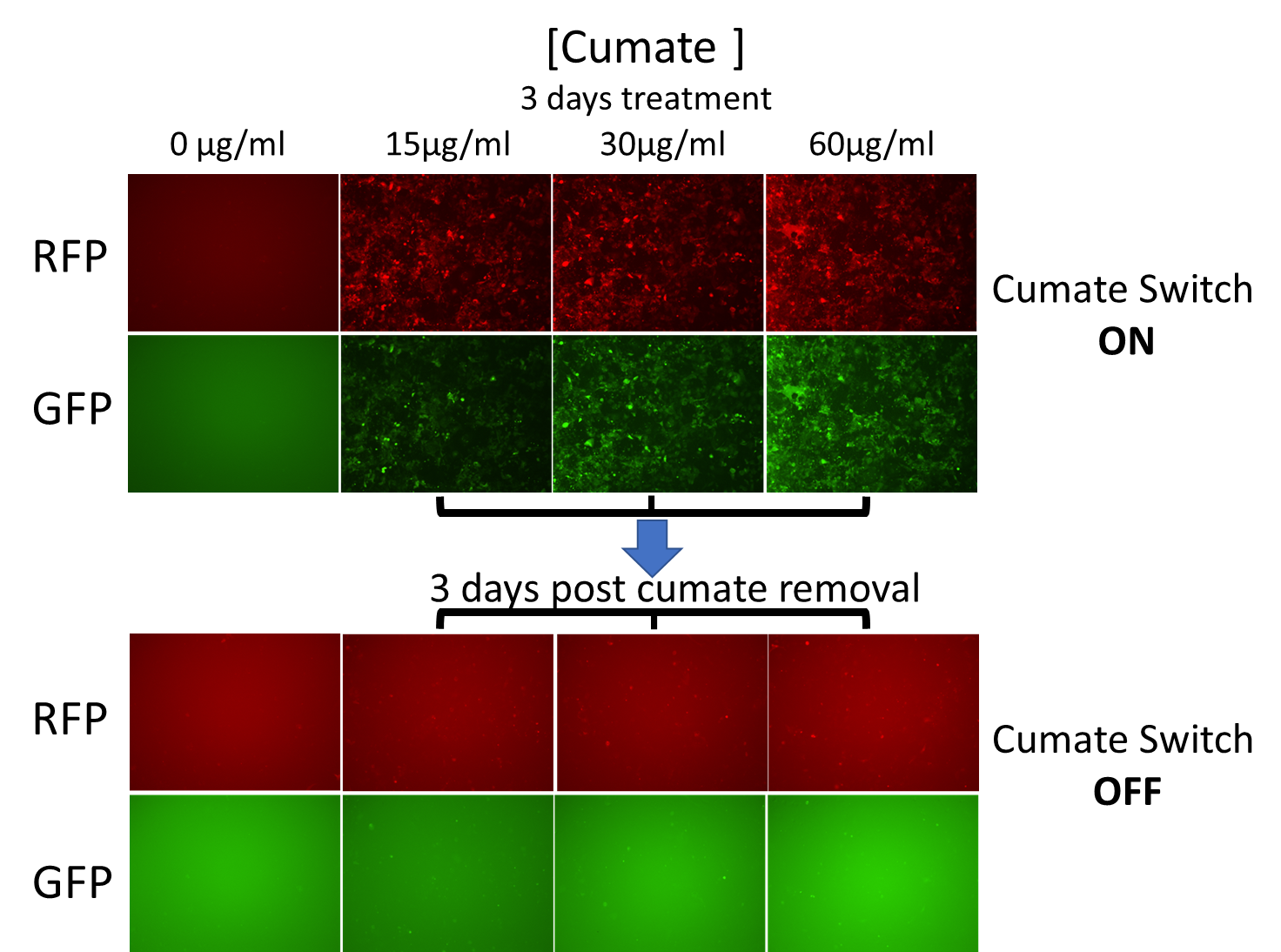
Figure 2 : QM826B-1 was packaged into virus and infected HEK293 cells at MOI of 15. 3 days after infection, 1ug/ml puromycin was added into culture medium to establish stable cell line. Then QM826B-1 HEK293 stable cell line was treated with cumate at difference concentration. 3 days after cumate treatment, RFP and GFP expression was well induced. Cumate was then removed from the culture medium. After 3 days of cumate-free, RFP and GFP expression was barely noticeable.
Figure 3. Gene expression with the SparQ™2 Cumate Switch System is titratable and reversible.
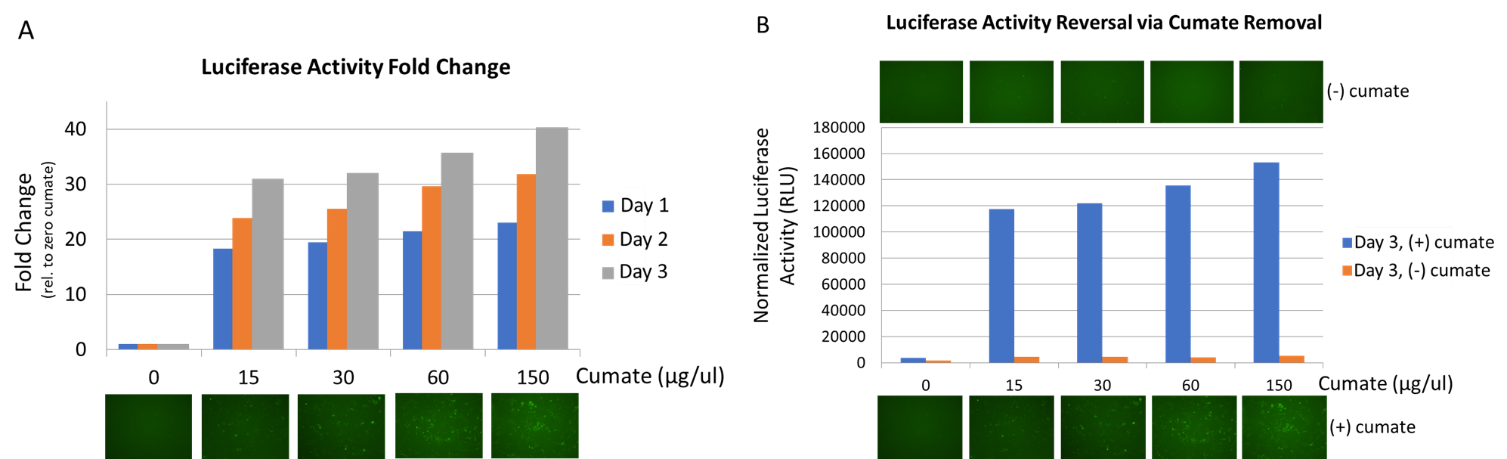
Figure 3 : We used QM828B-1 packaged into virus and infected HEK293 cells at MOI of 15. 3 days after infection, 1µg/ml puromycin was added into culture medium to establish stable cell line. (A) QM828B-1 HEK293 stable cell line was treated with cumate at different concentration. Cells were collected at day 1, day 2, and day 3 for luciferase assay to check the luciferase activity. SparQ Cumate Switch System can increase expression up to 40-fold after 3 days of cumate treatment. (B) To inactivate the gene transcription, cumate was removed from the culture medium. After 3 days without cumate treatment, Luciferase and GFP expressed was barely detected.
Figure 4. Gene expression with the SparQ™2 Cumate Switch System is reversible and repeatable.
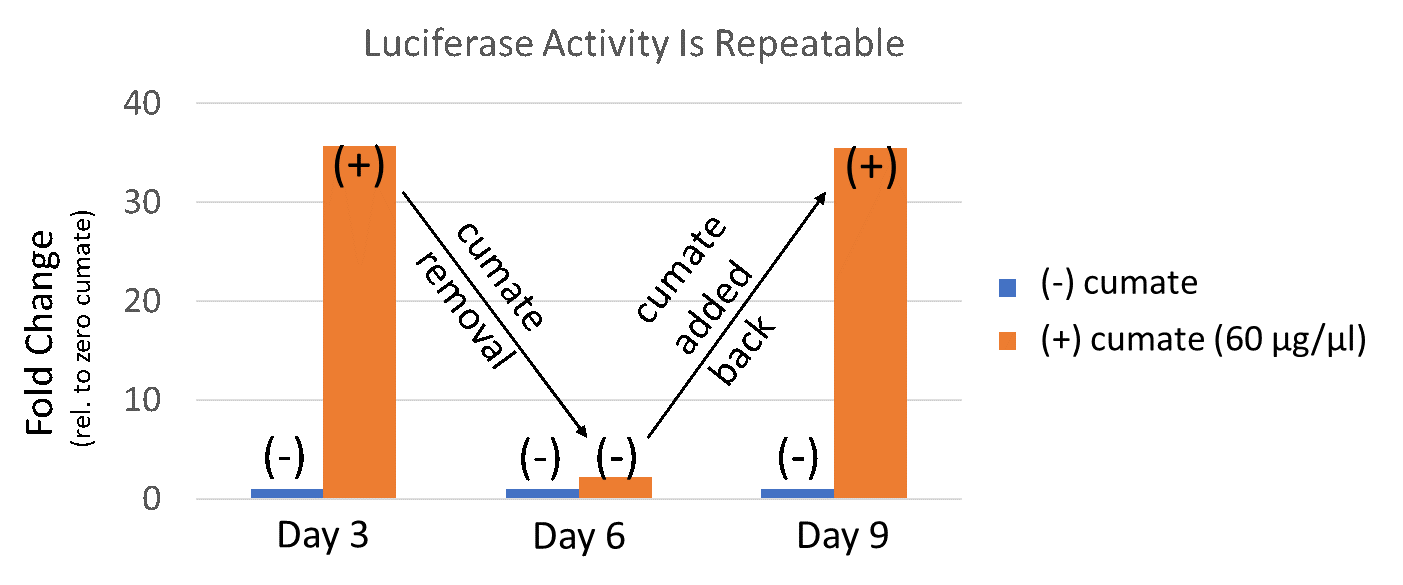
Figure 4 : QM828B-1 was packaged into virus and infected HEK293 cells at MOI of 15. 3 days after infection, 1µg/ml puromycin was added into culture medium to establish stable cell line. Then QM828B-1/HEK293 stable cell line was treated with cumate at 60µg/ul. 3 days after cumate treatment, luciferase expression was well induced. Then cumate was removed from the culture medium. After 3 days without cumate treatment, luciferase activity returned to the control level. Finally, when cumate was added back to the culture media, luciferase activity was once again induced.
FAQs
Documentation
Citations
Related Products
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| QM820B-1 | Plasmid; pCDH-CuO-MCS-P2A-GFP-EF1a-CymR-T2A-Puro | 10 µg | $1753 |
|
||||
| QM821B-1 | Plasmid; pCDH-CuO-MCS-P2A-RFP-EF1-CymR-T2A-Puro | 10 µg | $1753 |
|
||||
| QM822B-1 | Plasmid; pCDH-CuO-MCS-P2A-GFP-EF1-CymR-T2A-Blast | 10 µg | $1753 |
|
||||
| QM823B-1 | Plasmid; pCDH-CuO-MCS-P2A-RFP-EF1-CymR-T2A-Blast | 10 µg | $1753 |
|
||||
| QM824B-1 | Plasmid; pCDH-CuO-MCS-P2A-GFP-EF1-CymR-T2A-Neo | 10 µg | $1753 |
|
||||
| QM825B-1 | Plasmid; pCDH-CuO-MCS-P2A-RFP-EF1-CymR-T2A-Neo | 10 µg | $1753 |
|
||||
| QM826B-1 | Plasmid; pCDH-CuO-RFP-P2A-GFP-EF1-CymR-T2A-Puro | 10 µg | $1753 |
|
||||
| QM828B-1 | Plasmid; pCDH-CuO-FLuc-P2A-GFP-EF1-CymR-T2A-Puro | 10 µg | $1753 |
|
||||
Overview
With SBI’s SparQ™2 Cumate Switch System, you can get inducible gene expression in mammalian cells with a range of cloning and expression lentivectors. It simplifies your SparQ™ projects by delivering your gene-of-interest in the same vector that has CymR built-in (vector shown below). The vector drives your gene-of-interest and fluorescent markers (GFP, or RFP) with the inducible cumate switch promoter, and constitutively co-expresses CymR and a resistance marker (puromycin, blasticidin, or neomycin) from an EF1α promoter.

SBI’s SparQ™2 Cumate Switch System gives researchers the confidence when working with gene regulation in mammalian cells.
- Robustness - increase expression up to 40-fold
- Adjustable - tune expression levels by titrating the amount of cumate
- Reversible - turn expression on, off, then on again
- Simple - the all-in-one format allows co-expression of CymR and your gene-of-interest
- Powerful - suitable for in vivo applications
- Various marker selections (SparQ™2) - GFP or RFP in combination of puromycin, blasticidin, and neomycin
Overall, SparQ™ system is an excellent choice for achieving highest controlled levels of gene expression. It has negligible background expression in the absence of cumate and is a great approach to study cellular perturbation.
References
How It Works
The regulatory mechanisms of the bacterial operons cmt and cym have been engineered to regulate gene expression in mammalian cells. In the repressor configuration, regulation is mediated by strong binding of the CymR repressor to the Cumate operator site (CuO), which is downstream of the CMV5 promoter. Addition of cumate (depicted as yellow circle in the graphic scheme below), a non-toxic small molecule inducer, relieves the repression and drives gene activation; in this case, fluorescent proteins (RFP and GFP). Subsequent removal of cumate from the growth media reverts the activation process, rendering gene switches to off mode.
SBI’s SparQ Cumate Switch System can be generalized as follows.
- CymR, a repressor that binds to cumate operator sequences in the absence of cumate
- MCS, to clone-in your gene-of-interest
- Cumate inducible promoter with cumate operator sequences (CuO) upstream of the MCS, to inducibly control gene expression
- Variety of selection markers, to monitor the induction or select stable cell line
- CymR has a high binding affinity for cumate and, as more cumate is added, fewer CymR molecules bind to the CuO sequences in the promoter resulting in increased expression
- Exhibiting much lower background expression than similar systems
Figure 1. Cumate Switch System

Supporting Data
Tight expression control, low background, and reversible with the SparQ™2 Cumate Switch System
Figure 2. Gene expression with the SparQ™2 Cumate Switch On/Off System

Figure 2 : QM826B-1 was packaged into virus and infected HEK293 cells at MOI of 15. 3 days after infection, 1ug/ml puromycin was added into culture medium to establish stable cell line. Then QM826B-1 HEK293 stable cell line was treated with cumate at difference concentration. 3 days after cumate treatment, RFP and GFP expression was well induced. Cumate was then removed from the culture medium. After 3 days of cumate-free, RFP and GFP expression was barely noticeable.
Figure 3. Gene expression with the SparQ™2 Cumate Switch System is titratable and reversible.

Figure 3 : We used QM828B-1 packaged into virus and infected HEK293 cells at MOI of 15. 3 days after infection, 1µg/ml puromycin was added into culture medium to establish stable cell line. (A) QM828B-1 HEK293 stable cell line was treated with cumate at different concentration. Cells were collected at day 1, day 2, and day 3 for luciferase assay to check the luciferase activity. SparQ Cumate Switch System can increase expression up to 40-fold after 3 days of cumate treatment. (B) To inactivate the gene transcription, cumate was removed from the culture medium. After 3 days without cumate treatment, Luciferase and GFP expressed was barely detected.
Figure 4. Gene expression with the SparQ™2 Cumate Switch System is reversible and repeatable.

Figure 4 : QM828B-1 was packaged into virus and infected HEK293 cells at MOI of 15. 3 days after infection, 1µg/ml puromycin was added into culture medium to establish stable cell line. Then QM828B-1/HEK293 stable cell line was treated with cumate at 60µg/ul. 3 days after cumate treatment, luciferase expression was well induced. Then cumate was removed from the culture medium. After 3 days without cumate treatment, luciferase activity returned to the control level. Finally, when cumate was added back to the culture media, luciferase activity was once again induced.

