CD9 Exo-Flow Capture Kit
- A range of well-validated antibodies for reliable and reproducible purification
- Large-sized magnetic beads increase the efficiency of exosome capture
- Reversible Exo-FITC and Exo-APC stains can be completely removed after FACS
- Exosome Elution Buffer simultaneously removes Exo-FITC (or Exo-APC) and elutes exosomes from the magnetic beads for downstream applications such as functional studies
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXOFLOW100A-1 | CD9 Exo-Flow capture kit (Magnetic streptavidin beads, CD9-biotin capture antibody, Wash and Elution Buffers, Exo-FITC stain) | 10 Reactions | $519 |
|
||||
Overview
Overview
Go with the flow for easy exosome isolation using FACS
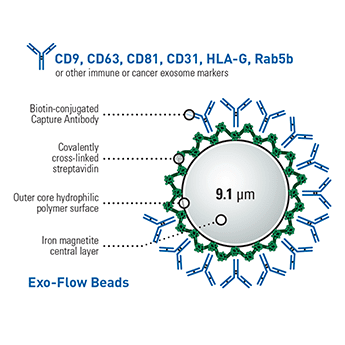
The CD9 Exo-Flow Capture Kit has all the reagents you need to purify exosomes using FACS—magnetic streptavidin beads, CD9-biotin capture antibody, wash and elution buffers, and reversible Exo-FITC stain (Cat.# EXOFLOW800A-1)—you supply the FACS and the optional magnetic stand (Cat.# EXOFLOW700A-1). Our well-validated CD9-biotin capture antibody and high-quality kit components ensure reliable, reproducible CD9-based exosome purification. And with our larger-than-typical bead size (9.1 μm diameter) exosome capture is highly efficient, assisting with recovery of rare exosome populations.
Note that we also offer reversible Exo-APC (Cat.# Exo-FLOW810A-1) as an alternative stain to Exo-FITC.- A range of well-validated antibodies for reliable and reproducible purification
- Large-sized magnetic beads increase the efficiency of exosome capture
- Reversible Exo-FITC and Exo-APC stains can be completely removed after FACS
- Exosome Elution Buffer simultaneously removes Exo-FITC (or Exo-APC) and elutes exosomes from the magnetic beads for downstream applications such as functional studies
More than just CD9
To facilitate the widest range of studies, SBI built the Exo-Flow system to be highly modular. We offer a range of biotinylated antibodies as well as a Basic Exo-Flow Kit (Cat.# CSFLOWBASICA-1) with no antibody so that you can use your own biotinylated antibodies.| Cat.# | Kit |
|---|---|
| EXOFLOW100A-1 | CD9 Exo-Flow Capture Kit |
| EXOFLOW150A-1 | Tetraspanin Exo-Flow Combo Capture Kit |
| EXOFLOW200A-1 | CD31 Exo-Flow Capture Kit |
| EXOFLOW210A-1 | CD44 Exo-Flow Capture Kit |
| EXOFLOW300A-1 | CD63 Exo-Flow Capture Kit |
| EXOFLOW400A-1 | CD81 Exo-Flow Capture Kit |
| EXOFLOW500A-1 | Rab5b Exo-Flow Capture Kit |
| EXOFLOW600A-1 | HLA-G Exo-Flow Capture Kit |
| EXOFLOW610A-1 | CD14 Exo-Flow Capture Kit |
| EXOFLOW620A-1 | CD68 Exo-Flow Capture Kit |
| EXOFLOW630A-1 | EpCAM Exo-Flow Capture Kit |
| EXOFLOW660A-1 | CD73 Exo-Flow Capture Kit |
How It Works
How It Works
Easily purify exosomes using FACS with the CD9 Exo-Flow Capture Kit
At a glance
Simply (1) couple the CD9-biotin antibody to the magnetic streptavidin beads, (2) use the CD-9-coupled magnetic beads to capture exosomes that have been isolated using either ExoQuick® or ultracentrifugation, (3) wash away unbound exosomes, and then (4) stain with reversible Exo-FITC (excitation and emission wavelengths of 494 nm and 518 nm, respectively).
Your sample is now ready for FACS analysis.
To use the purified exosomes after FACS, add the included Exosome Elution Buffer to simultaneously remove the Exo-FITC stain and elute intact exosomes from the beads.
Supporting Data
Supporting Data
See the CD9 Exo-FLOW Capture Kit in action
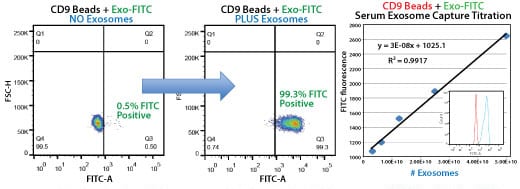
The CD9 Exo-Flow Capture Kit delivers quantitative, highly selective exosome isolation. Bead flow separation data for exosomes secreted by HEK293 cells and captured using the CD9 Exo-Flow Capture Kit. Plots of forward scatter versus FITC intensity show that in the no exosome control, only 0.5% of particles are FITC-positive (left panel), whereas in the exosome-containing sample, 99.3% of particles are FITC-positive (middle panel). The highly linear binding capacity of the Exo-Flow beads is shown (right panel), with the degree of flow separation shown in the inset graph.
Human serum exosomes were isolated from 250 µL serum using ExoQuick® and the exosome pellet resuspended in 500 µL of 1x PBS. Exosome particles were added as two-fold dilutions starting at 50 µL, and then captured using the biotinylated CD9 antibody coupled to Exo-Flow beads. The FITC flow cytometric intensities are then plotted versus the number of exosome particles (determined using NanoSight).
FAQs
Resources
Related Products
Citations
-
Boyer, E, et al. (2024) Comparison of plasma soluble and extracellular vesicles-associated biomarkers in Alzheimer’s Disease patients and cognitively normal individuals. medRxiv. 2024;. Link: medRxiv
-
Klemetti, MM, et al. (2024) Lipid profile of circulating placental extracellular vesicles during pregnancy identifies foetal growth restriction risk. Journal of extracellular vesicles. 2024; 13(2):e12413. PM ID: 38353485
-
Mekala, N, et al. (2024) Alcohol and e-cigarette damage alveolar-epithelial barrier by activation of P2X7r and provoke brain endothelial injury via extracellular vesicles. Cell communication and signaling : CCS. 2024; 22(1):39. PM ID: 38225580
-
Garbin, A, et al. (2023) MiR-146a-5p enrichment in small-extracellular vesicles of relapsed pediatric ALCL patients promotes macrophages infiltration and differentiation. Biochemical pharmacology. 2023; 215:115747. PM ID: 37591448
-
Guda, P, et al. (2023) Nanoscopic and functional characterization of keratinocyte-originating exosomes in the wound fluid of non-diabetic and diabetic chronic wound patients. Nano Today. 2023; 52:101954. Link: Nano Today
-
Nakazaki, M, et al. (2023) Human mesenchymal stem-derived extracellular vesicles improve body growth and motor function following severe spinal cord injury in rat. Clinical and translational medicine. 2023; 13(6):e1284. PM ID: 37323108
-
Mimmi, S, et al. (2023) SARS CoV-2 spike protein-guided exosome isolation facilitates detection of potential miRNA biomarkers in COVID-19 infections. Clinical chemistry and laboratory medicine. 2023;. PM ID: 36972680
-
Bertolini, I, et al. (2023) Intercellular HIF1a reprogams mammary progenitors and myeloid immune evasion to drive high-risk breast lesions. The Journal of clinical investigation. 2023;. PM ID: 36892943
-
Shen, H & Lane, RA. (2023) Extracellular Vesicles from Inflammation-Primed Adipose-Derived Stem Cells Enhance Achilles Tendon Repair by Reducing Inflammation and Promoting Intrinsic Healing. bioRxiv : the preprint server for biology. 2023;. PM ID: 36778262
-
Yuan, X, et al. (2023) Contribution of Hepatic Steatosis-Intensified Extracellular Vesicle Release to Aggravated Inflammatory Endothelial Injury in Liver-Specific Asah1 Gene Knockout Mice. The American journal of pathology. 2023;. PM ID: 36638912
-
Koken, GY, et al. (2022) Wharton Jelly Derived Mesenchymal Stem Cell’s Exosomes Demonstrate Significant Antileishmanial and Wound Healing Effects in Combination with Aloe-Emodin: An in Vitro Study. Journal of pharmaceutical sciences. 2022;. PM ID: 35995206
-
Luo, Z, et al. (2022) Human bone marrow mesenchymal stem cell-derived extracellular vesicles inhibit shoulder stiffness via let-7a/Tgfbr1 axis. Bioactive Materials. 2022;. Link: Bioactive Materials
-
Nirujogi, TS, et al. (2022) Lipidomic Profiling of Bronchoalveolar Lavage Fluid Extracellular Vesicles Indicates Their Involvement in Lipopolysaccharide-Induced Acute Lung Injury. Journal of innate immunity. 2022;:1-14. PM ID: 35367992
-
Marra, KV, et al. (2022) Bioactive extracellular vesicles from a subset of endothelial progenitor cells rescue retinal ischemia and neurodegeneration. JCI insight. 2022; 7(12). PM ID: 35639473
-
Li, Y, et al. (2022) Exosome-shuttled miR-126 mediates ethanol-induced disruption of neural crest cell-placode cell interaction by targeting SDF1. Research Square. 2022;. Link: Research Square
-
Mullen, M, et al. (2022) Mechanical strain drives exosome production, function, and miRNA cargo in C2C12 muscle progenitor cells. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2022;. PM ID: 36250617
-
Beatriz, M, et al. (2022) Defective mitochondria‐lysosomal axis enhances the release of extracellular vesicles containing mitochondrial DNA and proteins in Huntington’s disease. Journal of Extracellular Biology. 2022; 1(10). Link: Journal of Extracellular Biology
-
Zhang, H, et al. (2021) Improving Isolation of Extracellular Vesicles by Utilizing Nanomaterials. Membranes. 2021; 12(1). PM ID: 35054584
-
Luo, ZW, et al. (2021) Exosomes derived from inflammatory myoblasts promote M1 polarization and break the balance of myoblast proliferation/differentiation. World journal of stem cells. 2021; 13(11):1762-1782. PM ID: 34909122
-
Zhang, Z, et al. (2021) Surface Located Adiponectin On Adipocytes-derived Exosomes Mediates Adipocytes/cardiomyocytes Communication And Contributes To Cardioprotection. Circulation. 2021;. Link: Circulation
- See More
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXOFLOW100A-1 | CD9 Exo-Flow capture kit (Magnetic streptavidin beads, CD9-biotin capture antibody, Wash and Elution Buffers, Exo-FITC stain) | 10 Reactions | $519 |
|
||||
Overview
Overview
Go with the flow for easy exosome isolation using FACS
The CD9 Exo-Flow Capture Kit has all the reagents you need to purify exosomes using FACS—magnetic streptavidin beads, CD9-biotin capture antibody, wash and elution buffers, and reversible Exo-FITC stain (Cat.# EXOFLOW800A-1)—you supply the FACS and the optional magnetic stand (Cat.# EXOFLOW700A-1). Our well-validated CD9-biotin capture antibody and high-quality kit components ensure reliable, reproducible CD9-based exosome purification. And with our larger-than-typical bead size (9.1 μm diameter) exosome capture is highly efficient, assisting with recovery of rare exosome populations.
Note that we also offer reversible Exo-APC (Cat.# Exo-FLOW810A-1) as an alternative stain to Exo-FITC.- A range of well-validated antibodies for reliable and reproducible purification
- Large-sized magnetic beads increase the efficiency of exosome capture
- Reversible Exo-FITC and Exo-APC stains can be completely removed after FACS
- Exosome Elution Buffer simultaneously removes Exo-FITC (or Exo-APC) and elutes exosomes from the magnetic beads for downstream applications such as functional studies

More than just CD9
To facilitate the widest range of studies, SBI built the Exo-Flow system to be highly modular. We offer a range of biotinylated antibodies as well as a Basic Exo-Flow Kit (Cat.# CSFLOWBASICA-1) with no antibody so that you can use your own biotinylated antibodies.| Cat.# | Kit |
|---|---|
| EXOFLOW100A-1 | CD9 Exo-Flow Capture Kit |
| EXOFLOW150A-1 | Tetraspanin Exo-Flow Combo Capture Kit |
| EXOFLOW200A-1 | CD31 Exo-Flow Capture Kit |
| EXOFLOW210A-1 | CD44 Exo-Flow Capture Kit |
| EXOFLOW300A-1 | CD63 Exo-Flow Capture Kit |
| EXOFLOW400A-1 | CD81 Exo-Flow Capture Kit |
| EXOFLOW500A-1 | Rab5b Exo-Flow Capture Kit |
| EXOFLOW600A-1 | HLA-G Exo-Flow Capture Kit |
| EXOFLOW610A-1 | CD14 Exo-Flow Capture Kit |
| EXOFLOW620A-1 | CD68 Exo-Flow Capture Kit |
| EXOFLOW630A-1 | EpCAM Exo-Flow Capture Kit |
| EXOFLOW660A-1 | CD73 Exo-Flow Capture Kit |
How It Works
How It Works
Easily purify exosomes using FACS with the CD9 Exo-Flow Capture Kit
At a glance
Simply (1) couple the CD9-biotin antibody to the magnetic streptavidin beads, (2) use the CD-9-coupled magnetic beads to capture exosomes that have been isolated using either ExoQuick® or ultracentrifugation, (3) wash away unbound exosomes, and then (4) stain with reversible Exo-FITC (excitation and emission wavelengths of 494 nm and 518 nm, respectively).
Your sample is now ready for FACS analysis.
To use the purified exosomes after FACS, add the included Exosome Elution Buffer to simultaneously remove the Exo-FITC stain and elute intact exosomes from the beads.
Supporting Data
Supporting Data
See the CD9 Exo-FLOW Capture Kit in action
The CD9 Exo-Flow Capture Kit delivers quantitative, highly selective exosome isolation. Bead flow separation data for exosomes secreted by HEK293 cells and captured using the CD9 Exo-Flow Capture Kit. Plots of forward scatter versus FITC intensity show that in the no exosome control, only 0.5% of particles are FITC-positive (left panel), whereas in the exosome-containing sample, 99.3% of particles are FITC-positive (middle panel). The highly linear binding capacity of the Exo-Flow beads is shown (right panel), with the degree of flow separation shown in the inset graph.
Human serum exosomes were isolated from 250 µL serum using ExoQuick® and the exosome pellet resuspended in 500 µL of 1x PBS. Exosome particles were added as two-fold dilutions starting at 50 µL, and then captured using the biotinylated CD9 antibody coupled to Exo-Flow beads. The FITC flow cytometric intensities are then plotted versus the number of exosome particles (determined using NanoSight).
FAQs
Citations
-
Boyer, E, et al. (2024) Comparison of plasma soluble and extracellular vesicles-associated biomarkers in Alzheimer’s Disease patients and cognitively normal individuals. medRxiv. 2024;. Link: medRxiv
-
Klemetti, MM, et al. (2024) Lipid profile of circulating placental extracellular vesicles during pregnancy identifies foetal growth restriction risk. Journal of extracellular vesicles. 2024; 13(2):e12413. PM ID: 38353485
-
Mekala, N, et al. (2024) Alcohol and e-cigarette damage alveolar-epithelial barrier by activation of P2X7r and provoke brain endothelial injury via extracellular vesicles. Cell communication and signaling : CCS. 2024; 22(1):39. PM ID: 38225580
-
Garbin, A, et al. (2023) MiR-146a-5p enrichment in small-extracellular vesicles of relapsed pediatric ALCL patients promotes macrophages infiltration and differentiation. Biochemical pharmacology. 2023; 215:115747. PM ID: 37591448
-
Guda, P, et al. (2023) Nanoscopic and functional characterization of keratinocyte-originating exosomes in the wound fluid of non-diabetic and diabetic chronic wound patients. Nano Today. 2023; 52:101954. Link: Nano Today
-
Nakazaki, M, et al. (2023) Human mesenchymal stem-derived extracellular vesicles improve body growth and motor function following severe spinal cord injury in rat. Clinical and translational medicine. 2023; 13(6):e1284. PM ID: 37323108
-
Mimmi, S, et al. (2023) SARS CoV-2 spike protein-guided exosome isolation facilitates detection of potential miRNA biomarkers in COVID-19 infections. Clinical chemistry and laboratory medicine. 2023;. PM ID: 36972680
-
Bertolini, I, et al. (2023) Intercellular HIF1a reprogams mammary progenitors and myeloid immune evasion to drive high-risk breast lesions. The Journal of clinical investigation. 2023;. PM ID: 36892943
-
Shen, H & Lane, RA. (2023) Extracellular Vesicles from Inflammation-Primed Adipose-Derived Stem Cells Enhance Achilles Tendon Repair by Reducing Inflammation and Promoting Intrinsic Healing. bioRxiv : the preprint server for biology. 2023;. PM ID: 36778262
-
Yuan, X, et al. (2023) Contribution of Hepatic Steatosis-Intensified Extracellular Vesicle Release to Aggravated Inflammatory Endothelial Injury in Liver-Specific Asah1 Gene Knockout Mice. The American journal of pathology. 2023;. PM ID: 36638912
-
Koken, GY, et al. (2022) Wharton Jelly Derived Mesenchymal Stem Cell’s Exosomes Demonstrate Significant Antileishmanial and Wound Healing Effects in Combination with Aloe-Emodin: An in Vitro Study. Journal of pharmaceutical sciences. 2022;. PM ID: 35995206
-
Luo, Z, et al. (2022) Human bone marrow mesenchymal stem cell-derived extracellular vesicles inhibit shoulder stiffness via let-7a/Tgfbr1 axis. Bioactive Materials. 2022;. Link: Bioactive Materials
-
Nirujogi, TS, et al. (2022) Lipidomic Profiling of Bronchoalveolar Lavage Fluid Extracellular Vesicles Indicates Their Involvement in Lipopolysaccharide-Induced Acute Lung Injury. Journal of innate immunity. 2022;:1-14. PM ID: 35367992
-
Marra, KV, et al. (2022) Bioactive extracellular vesicles from a subset of endothelial progenitor cells rescue retinal ischemia and neurodegeneration. JCI insight. 2022; 7(12). PM ID: 35639473
-
Li, Y, et al. (2022) Exosome-shuttled miR-126 mediates ethanol-induced disruption of neural crest cell-placode cell interaction by targeting SDF1. Research Square. 2022;. Link: Research Square
-
Mullen, M, et al. (2022) Mechanical strain drives exosome production, function, and miRNA cargo in C2C12 muscle progenitor cells. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2022;. PM ID: 36250617
-
Beatriz, M, et al. (2022) Defective mitochondria‐lysosomal axis enhances the release of extracellular vesicles containing mitochondrial DNA and proteins in Huntington’s disease. Journal of Extracellular Biology. 2022; 1(10). Link: Journal of Extracellular Biology
-
Zhang, H, et al. (2021) Improving Isolation of Extracellular Vesicles by Utilizing Nanomaterials. Membranes. 2021; 12(1). PM ID: 35054584
-
Luo, ZW, et al. (2021) Exosomes derived from inflammatory myoblasts promote M1 polarization and break the balance of myoblast proliferation/differentiation. World journal of stem cells. 2021; 13(11):1762-1782. PM ID: 34909122
-
Zhang, Z, et al. (2021) Surface Located Adiponectin On Adipocytes-derived Exosomes Mediates Adipocytes/cardiomyocytes Communication And Contributes To Cardioprotection. Circulation. 2021;. Link: Circulation
- See More