EVs and cancer—current perspectives and research tools
Whether you are a cancer researcher who is new to EVs or have been actively researching EVs and cancer for some time, it can be tough to keep up with the latest findings. To help you out, we've compiled a list of resources that review current thoughts on EVs as well as the latest research tools.
A recent review
If you have time to read a review, there's a new open access article by Jie Dai, et al, that provides a concise overview of exosomes, some of the macromolecules they carry, the roles they play in signaling and cancer, and their potential use as therapeutics:
Exosomes: key players in cancer and potential therapeutic strategy
Dai J, et al. Signal Transduct Target Ther. 2020 Aug 5;5(1):145. DOI: 10.1038/s41392-020-00261-0
It should be noted that while Dai, et al, article focuses on nucleic acids and proteins, metabolites are also important EV cargo that can impact cancer physiology. To get a current overview of this topic, we recommend a review by Song Han, et al, which focuses on pancreatic ductal adenocarcinoma (PDAC).
From tumor microenvironment communicants to biomarker discovery: Selectively packaged extracellular vesicular cargoes in pancreatic cancer
Han S, et al. Cytokine Growth Factor Rev. 2020 Feb;51:61-68. doi: 10.1016/j.cytogfr.2020.01.001
A guided walk through a 2018 review
If you'd like a video overview of EVs and their therapeutic potential, we have a recent Tech Talk that incudes a nice introduction section with additional background on EVs before walking through their use in cancer therapeutics (skip to 4:05 to get to the start of the talk).
A twist on an established technique delivers a great new method for isolating EVs
As the field of EV research moves from being research use only to clinical applications, the question of the best way to isolate EVs for diagnostic and therapeutic use becomes more and more critical. Size exclusion chromatography (SEC) is rapidly becoming the method of choice (see Sidhom K, et al, Int J Mol Sci. 2020 Sep 4;21(18):E6466. doi: 10.3390/ijms21186466 for a recent review), but there are still drawbacks to the technology, including low yields and the need to concentrate the isolated EVs. Over the past year, SBI has released an enhanced SEC technology, the SmartSEC™ EV Isolation System, that delivers exceptionally clean and concentrated EVs. Keep reading to find out how, and/or download a PDF of our Technology Introduction.
Technology Introduction: The SmartSEC EV Isolation System
EVs are double-membrane vesicles in the size range of 30 nm to a few microns that are released from myriad cell types and are present in a variety of biofluids. These small entities are studied for biomarker development, cell communication, and targeted delivery. Rapid advances in EV research have generated a great demand for fast, robust, and reproducible EV isolation methods. Ultracentrifugation has been a commonly used isolation technique, but increasing revelations have shown the limitations and flaws of the approach, leading to the recent popularization of chromatography-based methods.
SEC is a classical technique used to separate molecules based on size, eluting the largest first and smallest last. In classical SEC, porous beads are well-packed into a long column for the separation of molecules. The sample is segregated into fractions, where EVs are separated from biomolecules of other sizes that elute in different fractions. The fractions can be analyzed for the presence or activity of the EVs.
Classical SEC has drawbacks, including collection of EV samples in multiple fractions and at low concentration, as well as the need for column packing and buffer-chase which adds to the processing time. To circumvent these issues, a next-generation size exclusion chromatography with smarter design – the SmartSEC isolation system - offers an approach that enables high efficiency purification where contaminants are trapped in the resin and EVs are eluted in one fraction rather than diluted into several fractions.
Advantages of SmartSEC
SmartSEC is a new generation of mixed-mode chromatography which provides the dual functionality of size exclusion and affinity interaction modes. Combining the two modes within a single resin media eliminates the time consuming and labor-intensive processes of classical SEC. With SmartSEC, the inside core of the porous bead is functionalized with affinity interacting modes, which enables the capture and retention of protein impurities up to 400 kDa (15-20 nm), such as IgG and albumin (Figure 1). The outside shell of the bead is inert, which minimizes nonspecific binding.
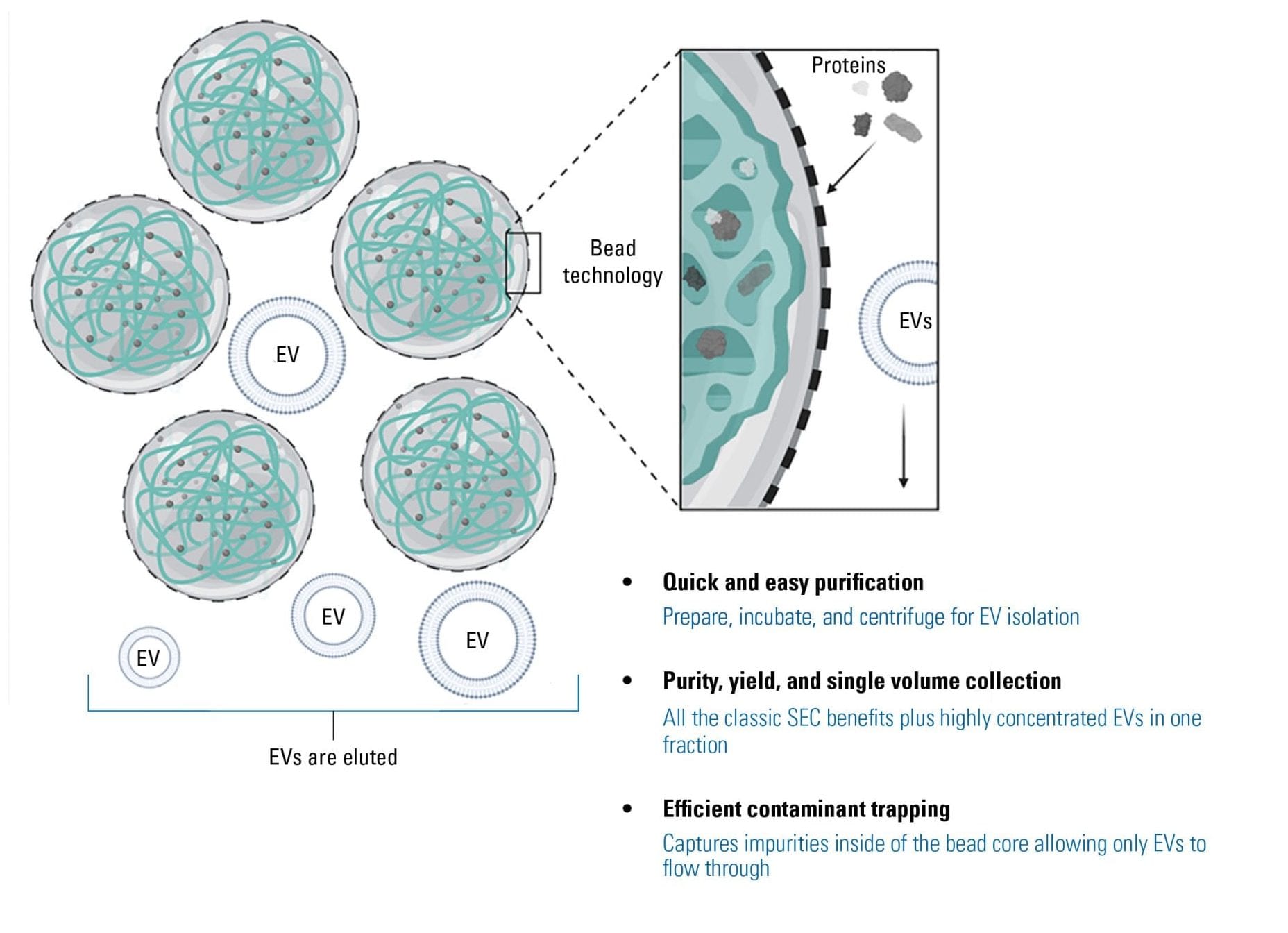
SmartSEC can function with superior binding capacity in an array of solutions, from tissue culture medium to serum or plasma.
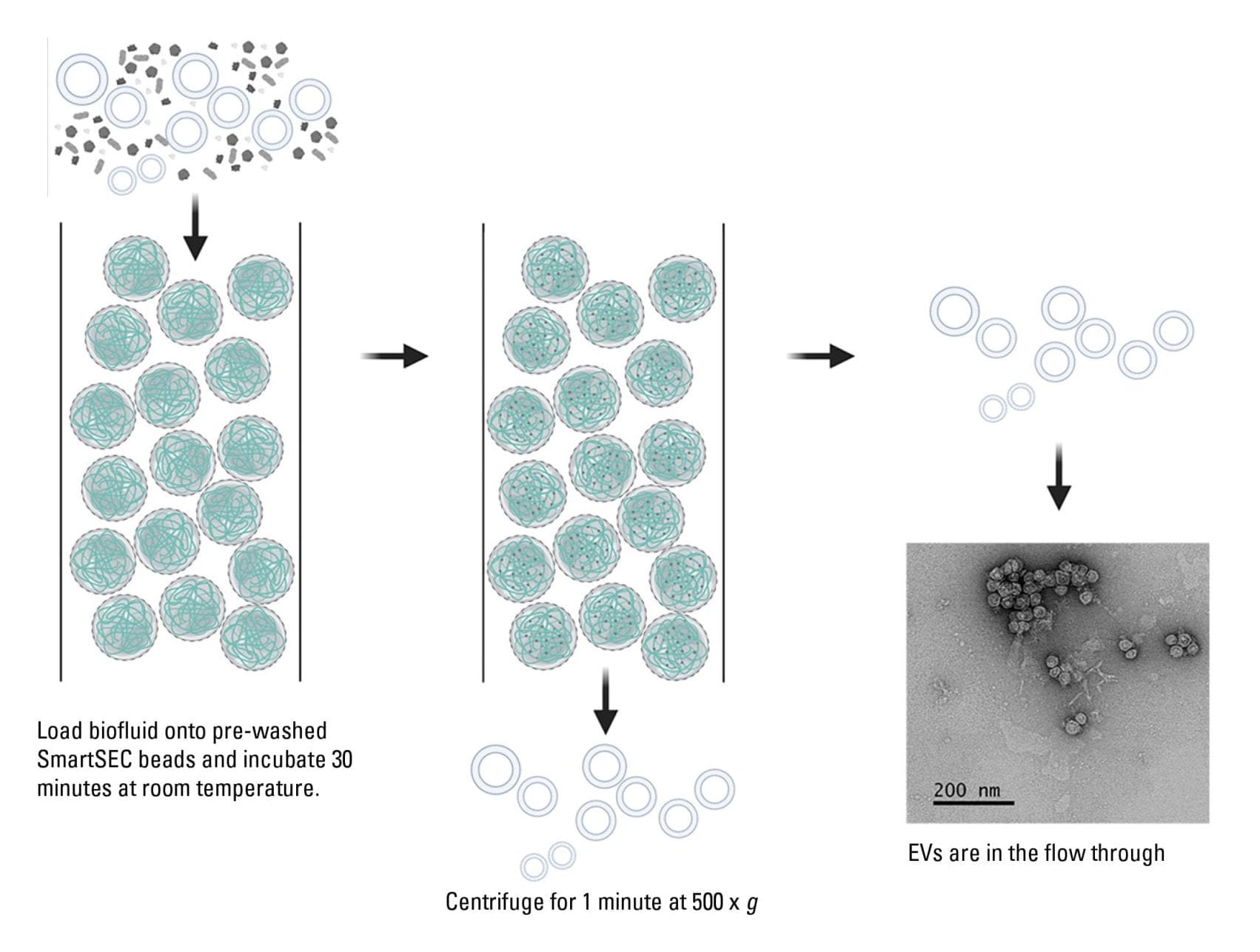
The SmartSEC column has been optimized to elute EVs at high concentration in one flow-through fraction as opposed to the collection of multiple low-concentration fractions in the classical SEC (Figure 2). To maximize the usefulness of the SmartSEC system, the technology was adapted into three formats to address the researchers’ needs for a wide range of sample types.
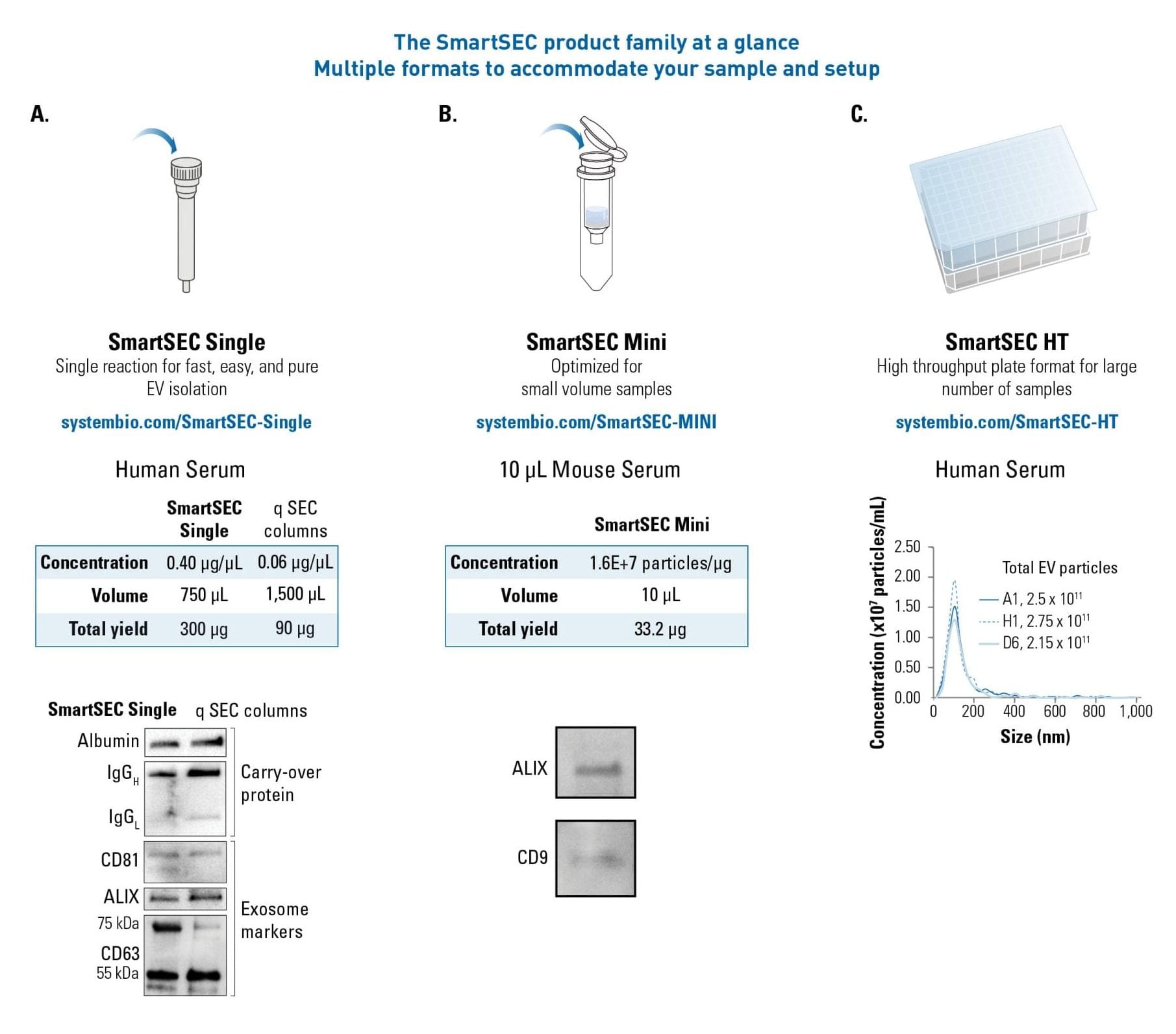
Different formats adapted for different samples
SmartSEC is available in three formats to suit a range of samples (Figure 3); it is user-friendly, time-saving, and generates higher yields of EVs than competitor SEC columns.
SmartSEC Single is designed for individual purification reaction of EVs from common samples such as human serum.
SmartSEC Mini is tailored for EV isolation from small volumes (10-100 μL) of precious samples.
Many of the current methods of EV isolation pose a limitation for those with a large number of samples. To address this need, SmartSEC-HT is a plate format developed to process up to 96 samples simultaneously. The SmartSEC product family can be easily adapted to most laboratories for accurate, reliable, and easy purification of EVs for downstream applications.
In all, the SmartSEC EV isolation technology offers an unprecedented way to isolate exosomes in workflows that were once deemed difficult; it has been proven to be user-friendly, time saving, and produces a high yield of pure/concentrated exosomes that is unattainable with traditional SEC methods.