EV-Guard™ EV Storage Buffer
- Ease of Use - Simply resuspend EV pellets in the 1x EV-GuardTM buffer or add the 10X concentrated EV-GuardTM buffer directly to the EV fractions from size exclusion column, making it a quick and easy step to preserve your EVs.
- Superior Protection – Achieve up to 98% EV conservation, significantly higher than with PBS, even after multiple freeze-thaw cycles, ensuring your EVs are well preserved and retained for your downstream applications.
- Non-Polymer Based and Non-Penetrating Cryoprotectant (CPA)
- Preserve Functionality – EVs have better transfection efficiency in EV-GuardTM than in PBS after multiple freeze-thaw cycles and long-term storage, assuring your EVs remain biologically active and ready to use.
- Downstream Applications Compatibility - NTA, fNTA, Western Blotting, ELISA, qPCR, NGS, EV delivery/transfection to cell culture, etc.
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXSBA-1 | EV-GuardTM EV Storage Buffer – 1X | 40 mL | $387 |
|
||||
| EXSBA-10 | EV-GuardTM EV Storage Buffer – 10X concentrated | 4 mL | $387 |
|
||||
Overview
Extracellular vesicles play a crucial role in cell-to-cell communication, carrying various biomolecules such as proteins, lipids, and nucleic acids. They have gained significant interest in research and clinical applications, such as diagnostics, therapeutics, and drug delivery. Preservation of the physical and biological characteristics of EVs is crucial for their use in downstream applications, making storage a key consideration.
Typically, EVs are suspended in PBS and stored at -80°C for long-term storage, or at -20°C/4°C for short-term storage. However, these storage conditions may be insufficient to prevent damage to and fragmentation of EVs, which could alter their functional properties.
The EV-GuardTM EV Storage Buffer is specifically formulated to prevent EV degradation, ensuring their biological activities are well-maintained. This feature is critical to the success of downstream research and clinical endeavors. With EV-GuardTM, your precious EV samples are well-preserved and ready for use in various applications.
Invest in EV-GuardTM EV Storage Buffer today and protect your valuable EV samples for tomorrow’s discoveries.
References
How It Works
The buffer is designed in both 1X and 10X concentrated formats to suit the needs for customers who choose to use different EV isolation methods which result in concentrated EV pellets (e.g., by ultracentrifugation, ExoQuick or other precipitation-based methods) or EV suspensions (e.g., by SmartSEC or other size exclusion-based methods).
Using the EV-GuardTM EV storage buffer – 1X- Resuspend purified EV pellet in 100 ul - 500 ul of 1X EV-GuardTM EV Storage Buffer.
- Optional: Perform a protein quantitation assay (e.g., BCA or Qubit assay) to determine the amount of protein in your sample.
- Aliquot EV samples into appropriate volumes for storage.
- Add 10X concentrated EV-GuardTM EV Storage Buffer to purified EV suspension to a final concentration of 1X. Gently pipet to mix.
- Optional: Perform a protein quantitation assay (e.g., BCA or Qubit assay) to determine the amount of protein in your sample.
- Aliquot EV samples into appropriate volumes for storage.
Supporting Data
EV-GuardTM EV Storage Buffer prevents loss of EV particles after multiple freeze-thaw cycles
EVs were isolated from HEK293T cell culture media using SBI’s ExoQuick-TC. The samples were stored at -80°C in either PBS or EV-GuardTM before undergoing single and multiple freeze-thaw cycles. The size distribution and concentrations of EV particles were determined by nanoparticle tracking analysis (NTA) (Figure 1A). Compared to the samples stored in PBS, which experienced approximately 30% loss after five freeze-thaw cycles, the EVs stored in EV-GuardTM exhibited 98% conservation (Figure 1B).
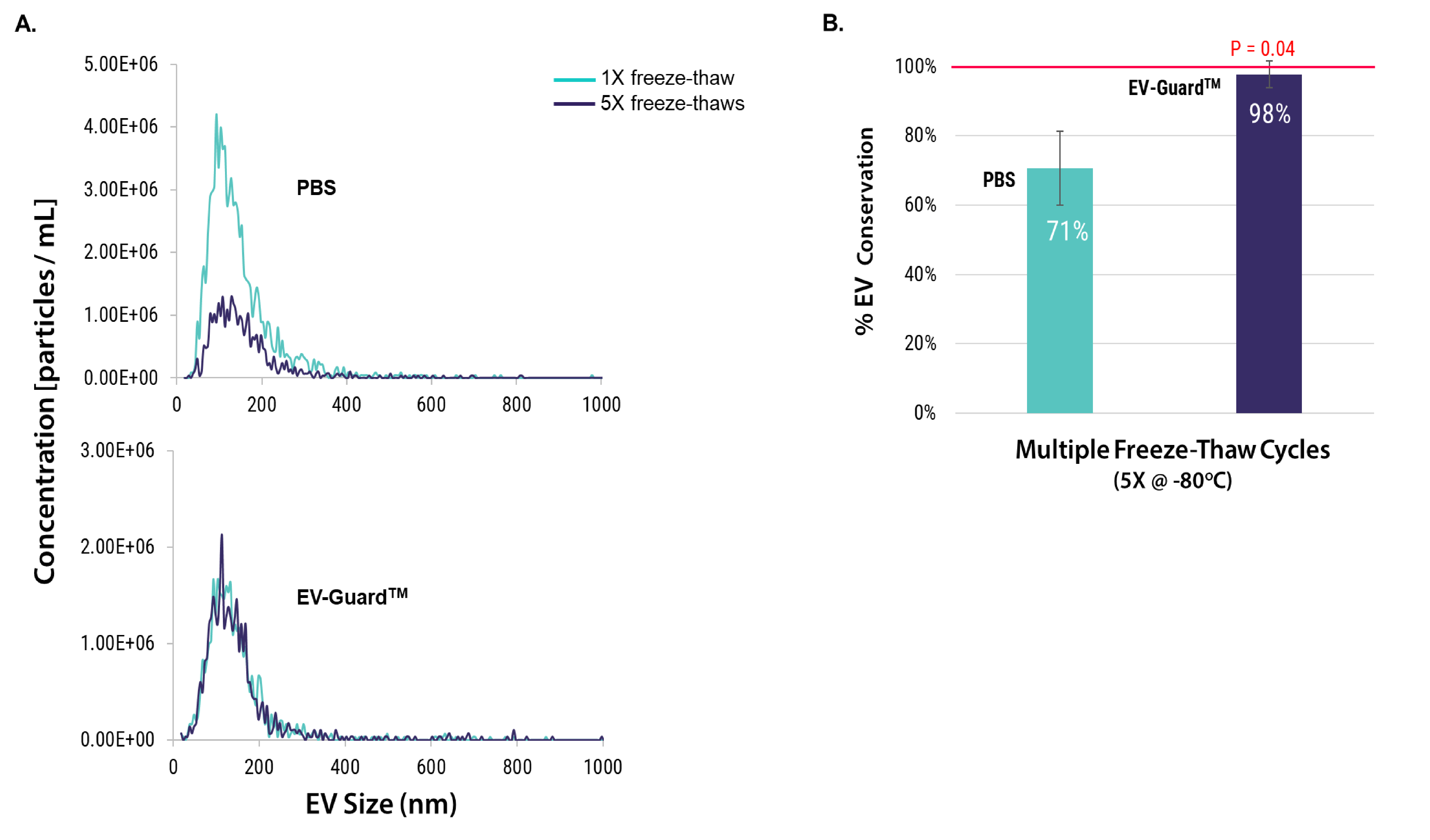
Figure 1: The size distribution (A) and the percentage of EV conservation (B) were assessed by NTA following multiple freeze-thaw cycles in both EV-GuardTM buffer and PBS. The data were derived from five independent experiments (n=5), each compared to a single freeze-thaw cycle.
EV-GuardTM EV Storage Buffer minimizes loss and aggregation of EV particles during long-term storage
EVs from normal human serum were isolated using SBI’s ExoQuick and stored at 4°C for intervals of 0 days, 15 days, and 30 days. Subsequent analyses were conducted using fluorescent nanoparticle tracking analysis (fNTA). It was observed that EV samples preserved in EV-GuardTM buffer exhibited a higher percentage of EV conservation than samples preserved in PBS after both 15 and 30 days (Figure 2A). Furthermore, the overall sizes of the EVs were consistently maintained during storage in EV-GuardTM, but showed variation when stored in PBS (Figure 2B).
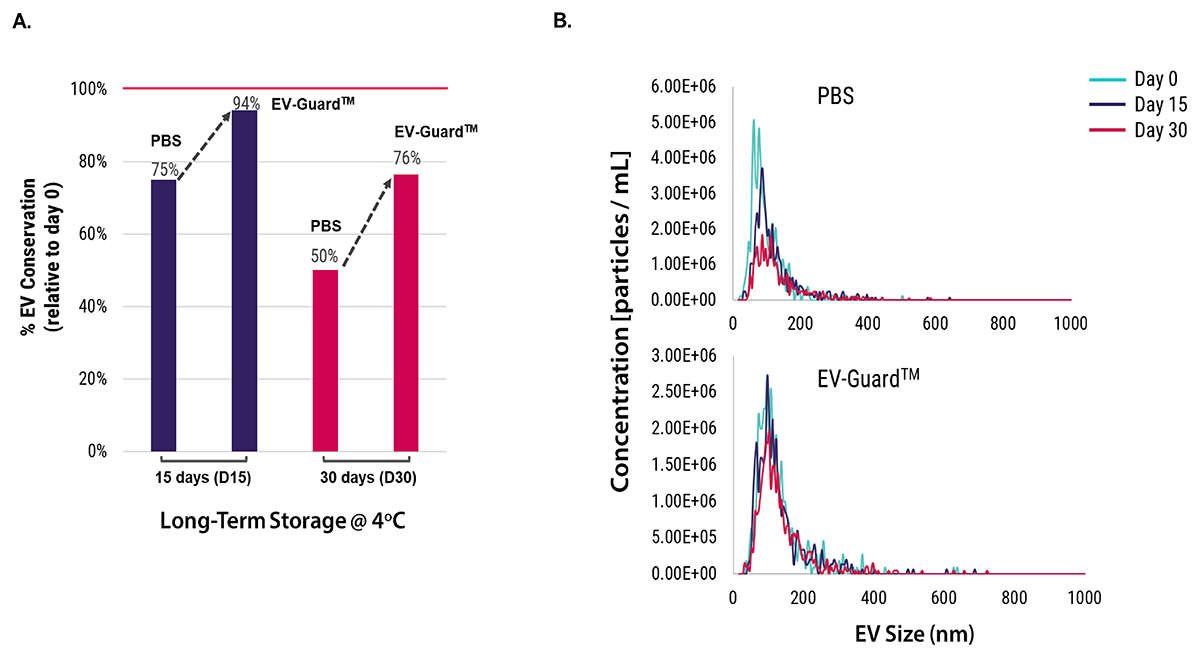
Figure 2: EVs from normal human serum were isolated using SBI’s ExoQuick and stored at 4°C for periods of 0, 15, and 30 days. The percentage of EV conservation (A) and EV size distribution (B) were subsequently analyzed using fluorescent nanoparticle tracking analysis (fNTA).
EV-GuardTM EV Storage Buffer preserves EV functionality and integrity after long-term storage
GFP-loaded EVs were isolated from the Xpack CMV-XP-GFP-EF1a-Puro Stable HEK293 Producer Cell Line (Cat. XPAK530CL-1). These EVs were stored in either PBS or EV-GuardTM at -20°C for 1 month before being introduced to HEK293 cells for transfection. The GFP-loaded EVs stored at -20°C in EV-GuardTM demonstrated higher transfection efficiency (Figure 3, C4) than those stored in PBS (Figure 3, C2). Moreover, the number of GFP+ EVs was higher in EV-GuardTM buffer compared to those stored in PBS after 1 month, as analyzed by fluorescent NTA (Figure 3 box).
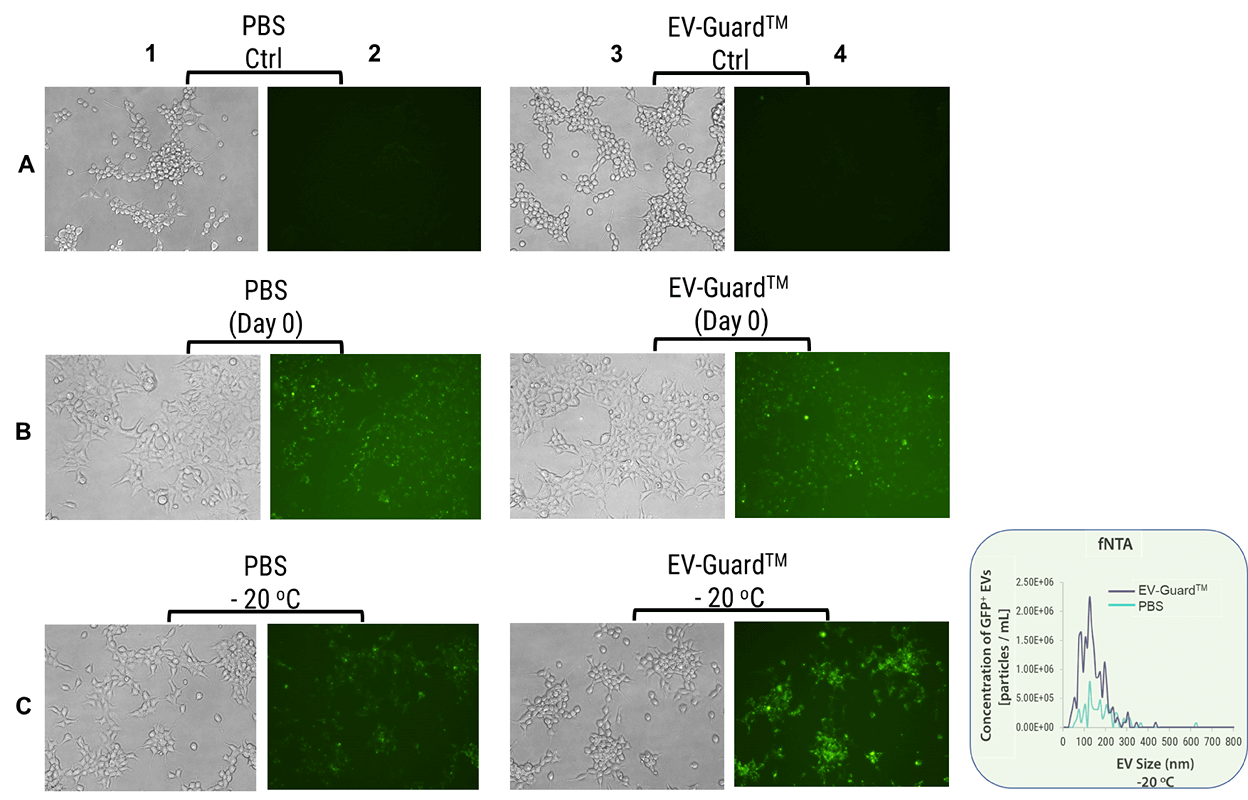
Figure 3: GFP-loaded EVs were stored in either PBS (columns 1 and 2) or EV-GuardTM (columns 3 and 4) at -20°C for 1 month before being introduced to HEK293 cells for transfection (Row C). Row A represents the negative control (no GFP EVs), while Row B is the positive control (no storage). After 1 month at -20°C, the number of GFP+ EVs was analyzed using fluorescent NTA (as shown in the box to the right).
FAQs
Documentation
Citations
Related Products
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXSBA-1 | EV-GuardTM EV Storage Buffer – 1X | 40 mL | $387 |
|
||||
| EXSBA-10 | EV-GuardTM EV Storage Buffer – 10X concentrated | 4 mL | $387 |
|
||||
Overview
Extracellular vesicles play a crucial role in cell-to-cell communication, carrying various biomolecules such as proteins, lipids, and nucleic acids. They have gained significant interest in research and clinical applications, such as diagnostics, therapeutics, and drug delivery. Preservation of the physical and biological characteristics of EVs is crucial for their use in downstream applications, making storage a key consideration.
Typically, EVs are suspended in PBS and stored at -80°C for long-term storage, or at -20°C/4°C for short-term storage. However, these storage conditions may be insufficient to prevent damage to and fragmentation of EVs, which could alter their functional properties.
The EV-GuardTM EV Storage Buffer is specifically formulated to prevent EV degradation, ensuring their biological activities are well-maintained. This feature is critical to the success of downstream research and clinical endeavors. With EV-GuardTM, your precious EV samples are well-preserved and ready for use in various applications.
Invest in EV-GuardTM EV Storage Buffer today and protect your valuable EV samples for tomorrow’s discoveries.
References
How It Works
The buffer is designed in both 1X and 10X concentrated formats to suit the needs for customers who choose to use different EV isolation methods which result in concentrated EV pellets (e.g., by ultracentrifugation, ExoQuick or other precipitation-based methods) or EV suspensions (e.g., by SmartSEC or other size exclusion-based methods).
Using the EV-GuardTM EV storage buffer – 1X- Resuspend purified EV pellet in 100 ul - 500 ul of 1X EV-GuardTM EV Storage Buffer.
- Optional: Perform a protein quantitation assay (e.g., BCA or Qubit assay) to determine the amount of protein in your sample.
- Aliquot EV samples into appropriate volumes for storage.
- Add 10X concentrated EV-GuardTM EV Storage Buffer to purified EV suspension to a final concentration of 1X. Gently pipet to mix.
- Optional: Perform a protein quantitation assay (e.g., BCA or Qubit assay) to determine the amount of protein in your sample.
- Aliquot EV samples into appropriate volumes for storage.
Supporting Data
EV-GuardTM EV Storage Buffer prevents loss of EV particles after multiple freeze-thaw cycles
EVs were isolated from HEK293T cell culture media using SBI’s ExoQuick-TC. The samples were stored at -80°C in either PBS or EV-GuardTM before undergoing single and multiple freeze-thaw cycles. The size distribution and concentrations of EV particles were determined by nanoparticle tracking analysis (NTA) (Figure 1A). Compared to the samples stored in PBS, which experienced approximately 30% loss after five freeze-thaw cycles, the EVs stored in EV-GuardTM exhibited 98% conservation (Figure 1B).

Figure 1: The size distribution (A) and the percentage of EV conservation (B) were assessed by NTA following multiple freeze-thaw cycles in both EV-GuardTM buffer and PBS. The data were derived from five independent experiments (n=5), each compared to a single freeze-thaw cycle.
EV-GuardTM EV Storage Buffer minimizes loss and aggregation of EV particles during long-term storage
EVs from normal human serum were isolated using SBI’s ExoQuick and stored at 4°C for intervals of 0 days, 15 days, and 30 days. Subsequent analyses were conducted using fluorescent nanoparticle tracking analysis (fNTA). It was observed that EV samples preserved in EV-GuardTM buffer exhibited a higher percentage of EV conservation than samples preserved in PBS after both 15 and 30 days (Figure 2A). Furthermore, the overall sizes of the EVs were consistently maintained during storage in EV-GuardTM, but showed variation when stored in PBS (Figure 2B).

Figure 2: EVs from normal human serum were isolated using SBI’s ExoQuick and stored at 4°C for periods of 0, 15, and 30 days. The percentage of EV conservation (A) and EV size distribution (B) were subsequently analyzed using fluorescent nanoparticle tracking analysis (fNTA).
EV-GuardTM EV Storage Buffer preserves EV functionality and integrity after long-term storage
GFP-loaded EVs were isolated from the Xpack CMV-XP-GFP-EF1a-Puro Stable HEK293 Producer Cell Line (Cat. XPAK530CL-1). These EVs were stored in either PBS or EV-GuardTM at -20°C for 1 month before being introduced to HEK293 cells for transfection. The GFP-loaded EVs stored at -20°C in EV-GuardTM demonstrated higher transfection efficiency (Figure 3, C4) than those stored in PBS (Figure 3, C2). Moreover, the number of GFP+ EVs was higher in EV-GuardTM buffer compared to those stored in PBS after 1 month, as analyzed by fluorescent NTA (Figure 3 box).

Figure 3: GFP-loaded EVs were stored in either PBS (columns 1 and 2) or EV-GuardTM (columns 3 and 4) at -20°C for 1 month before being introduced to HEK293 cells for transfection (Row C). Row A represents the negative control (no GFP EVs), while Row B is the positive control (no storage). After 1 month at -20°C, the number of GFP+ EVs was analyzed using fluorescent NTA (as shown in the box to the right).

