Genome Editing Positive Control EGIP 293T Cell Line
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| CAS606A-1 | Positive Control EGIP 293T Reporter Cell Line | 1 Vial | $753 |
|
||||
Overview
Overview
Confidence from the right controls
Use the Genome Editing Positive Control EGIP 293T Cell Line as a positive control for your genome editing projects (this is the same cell line provided in the Cas9 SmartNuclease™ Genome Editing Positive Control Kit).
The Genome Editing Positive Control EGIP 293T Reporter Cell Line has a non-fluorescent eGFP gene integrated into the genome, and is non-functional due to the insertion of a premature stop codon embedded in a 53 bp AAVS1 sequence. With an AAVS1-targeting gRNA, Cas9 (delivered as mRNA, lentivector, plasmid, or protein), and an appropriately-designed HR Targeting Vector, the premature stop codon can be removed, resulting in restoration of GFP fluorescence.
Note that because the control reporter cell line takes advantage of AAVS1 sequences, this cell line can also be used to verify AAVS1 targeting constructs.
How It Works
How It Works
Using the Genome Editing Positive Control EGIP 293T Cell Line
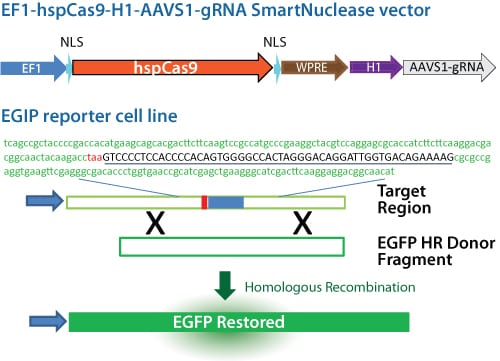
The Genome Editing Positive Control EGIP 293T Cell Line (kindly provided by Dr. Jizhong Zou of the NIH Center for Regenerative Medicine, a Common Fund initiative of the U.S. National Institutes of Health) can be used to verify genome editing techniques. This cell line contains a genomic copy of the EGFP gene that is non-fluorescent due to the insertion of a premature stop codon embedded in 53 bp of AAVS1 safe harbor site sequence (Figure 1).
Figure 1. Schematic of the All-in-one Cas9 SmartNuclease & AAVS1 gRNA Plasmid and Cas9 Positive Control EGIP 293T Reporter Cell Line.
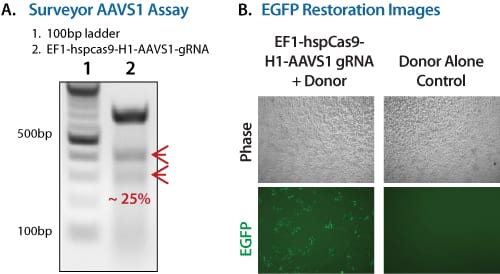
Co-transfection Cas9 and AAVS1 gRNA (delivered via the All-in-one Cas9 & AAVS1 gRNA plasmid) with an appropriate HR targeting vector results in repair of the eGFP gene and restoration of fluorescence (Figure 2B). The All-in-one Cas9 & AAVS1 gRNA plasmid delivers Cas9 activity that is targeted to AAVS1 sequences by the gRNA (Figure 2A).
Figure 2. Restoring GFP fluorescence with the Cas9 SmartNuclease Genome Editing Positive Control Kit. (A) Surveyor Assay data shows ~25% Cas9-mediated cleavage activity. (B) Fluorescence imaging shows restoration of GFP fluorescence only when the Cas9 SmartNuclease & AAVS1 gRNA Plasmid is included.
CRISPR/Cas9 Basics
Through careful selection of the target sequence and design of a donor plasmid for homologous recombination, you can achieve efficient and highly targeted genomic modification with CRISPR/Cas9.
The system
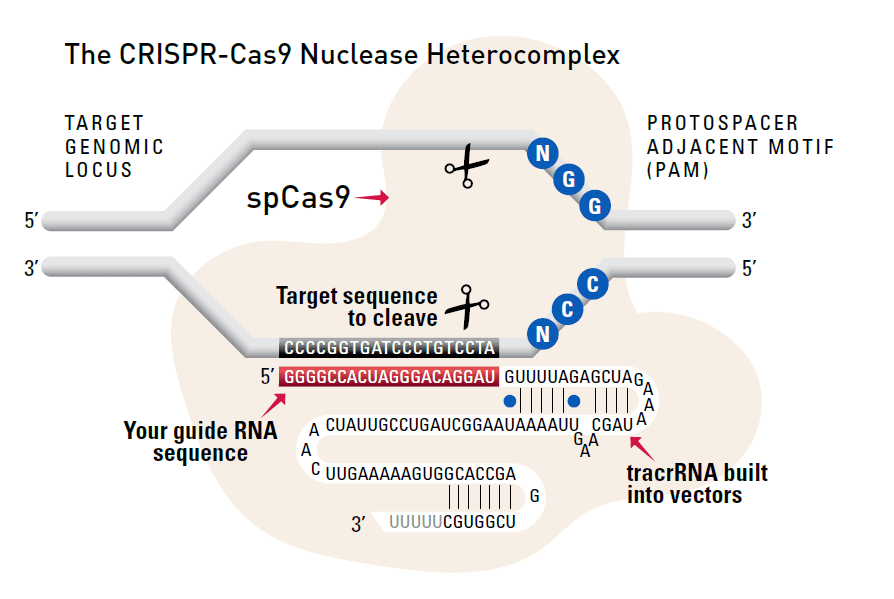
Cas9 protein—uses guide RNA (gRNA) to direct site-specific, double-strand DNA cleavage adjacent to a protospacer adapter motif (PAM) in the target DNA.
gRNA—RNA sequence that guides Cas9 to cleave a homologous region in the target genome. Efficient cleavage only where the gRNA homology is adjacent to a PAM.
PAM—protospacer adapter motif, NGG, is a target DNA sequence that spCas9 will cut upstream from if directed to by the gRNA.
The workflow at-a-glance
DESIGN: Select gRNA and HR donor plasmids. Choice of gRNA site and design of donor plasmid determines whether the homologous recombination event results in a knock-out, knock-in, edit, or tagging.
CONSTRUCT: Clone gRNA into all-in-one Cas9 vector. Clone 5’ and 3’ homology arms into HR donor plasmid. If creating a knock-in, clone desired gene into HR donor.
CO-TRANSFECT or CO-INJECT: Introduce Cas9, gRNA, and HR Donors into the target cells using co-transfection for plasmids, co-transduction for lentivirus, or co-injection for mRNAs.
SELECT/SCREEN: Select or screen for mutants and verify.
VALIDATE: Genotype or sequence putative mutants to verify single or biallelic conversion.
Supporting Data
Supporting Data
See the above “How it works” section
FAQs
Resources
Related Products
Citations
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| CAS606A-1 | Positive Control EGIP 293T Reporter Cell Line | 1 Vial | $753 |
|
||||
Overview
Overview
Confidence from the right controls
Use the Genome Editing Positive Control EGIP 293T Cell Line as a positive control for your genome editing projects (this is the same cell line provided in the Cas9 SmartNuclease™ Genome Editing Positive Control Kit).
The Genome Editing Positive Control EGIP 293T Reporter Cell Line has a non-fluorescent eGFP gene integrated into the genome, and is non-functional due to the insertion of a premature stop codon embedded in a 53 bp AAVS1 sequence. With an AAVS1-targeting gRNA, Cas9 (delivered as mRNA, lentivector, plasmid, or protein), and an appropriately-designed HR Targeting Vector, the premature stop codon can be removed, resulting in restoration of GFP fluorescence.
Note that because the control reporter cell line takes advantage of AAVS1 sequences, this cell line can also be used to verify AAVS1 targeting constructs.
How It Works
How It Works
Using the Genome Editing Positive Control EGIP 293T Cell Line
The Genome Editing Positive Control EGIP 293T Cell Line (kindly provided by Dr. Jizhong Zou of the NIH Center for Regenerative Medicine, a Common Fund initiative of the U.S. National Institutes of Health) can be used to verify genome editing techniques. This cell line contains a genomic copy of the EGFP gene that is non-fluorescent due to the insertion of a premature stop codon embedded in 53 bp of AAVS1 safe harbor site sequence (Figure 1).
Figure 1. Schematic of the All-in-one Cas9 SmartNuclease & AAVS1 gRNA Plasmid and Cas9 Positive Control EGIP 293T Reporter Cell Line.
Co-transfection Cas9 and AAVS1 gRNA (delivered via the All-in-one Cas9 & AAVS1 gRNA plasmid) with an appropriate HR targeting vector results in repair of the eGFP gene and restoration of fluorescence (Figure 2B). The All-in-one Cas9 & AAVS1 gRNA plasmid delivers Cas9 activity that is targeted to AAVS1 sequences by the gRNA (Figure 2A).
Figure 2. Restoring GFP fluorescence with the Cas9 SmartNuclease Genome Editing Positive Control Kit. (A) Surveyor Assay data shows ~25% Cas9-mediated cleavage activity. (B) Fluorescence imaging shows restoration of GFP fluorescence only when the Cas9 SmartNuclease & AAVS1 gRNA Plasmid is included.
CRISPR/Cas9 Basics
Through careful selection of the target sequence and design of a donor plasmid for homologous recombination, you can achieve efficient and highly targeted genomic modification with CRISPR/Cas9.
The system
Cas9 protein—uses guide RNA (gRNA) to direct site-specific, double-strand DNA cleavage adjacent to a protospacer adapter motif (PAM) in the target DNA.
gRNA—RNA sequence that guides Cas9 to cleave a homologous region in the target genome. Efficient cleavage only where the gRNA homology is adjacent to a PAM.
PAM—protospacer adapter motif, NGG, is a target DNA sequence that spCas9 will cut upstream from if directed to by the gRNA.
The workflow at-a-glance
DESIGN: Select gRNA and HR donor plasmids. Choice of gRNA site and design of donor plasmid determines whether the homologous recombination event results in a knock-out, knock-in, edit, or tagging.
CONSTRUCT: Clone gRNA into all-in-one Cas9 vector. Clone 5’ and 3’ homology arms into HR donor plasmid. If creating a knock-in, clone desired gene into HR donor.
CO-TRANSFECT or CO-INJECT: Introduce Cas9, gRNA, and HR Donors into the target cells using co-transfection for plasmids, co-transduction for lentivirus, or co-injection for mRNAs.
SELECT/SCREEN: Select or screen for mutants and verify.
VALIDATE: Genotype or sequence putative mutants to verify single or biallelic conversion.
Supporting Data
Supporting Data
See the above “How it works” section