SBI's Exosome Workshop Training Course
POSTPONED: Next Workshop - TBD

The next Exosome Workshop class will take place April 13 - 17, 2020 when circumstances allow
DUE TO THE COVID-19 PANDEMIC, SBI'S EXOSOME WORKSHOP WILL BE RESCHEUDLED FOR A LATER DATE
For more information, contact us at info@systembio.com
In this workshop, SBI's exosome experts will provide hands-on training on how to isolate, quantify, characterize, visualize, and engineer exosomes. Wet lab experience includes how to quantify isolated exosomes using enzymatic & ELISA assays, as well as Western blotting of common exosomal markers. Training on labeling exosomes to track cellular uptake and delivery as well as engineering exosome cargo will also be covered. This training course is designed for MS/PhD/MD/Postdoc students and scientists already in the exosome life science research field or those interested in beginning exosome studies. Previous knowledge in basic tissue culture is recommended.
What makes this workshop course unique?
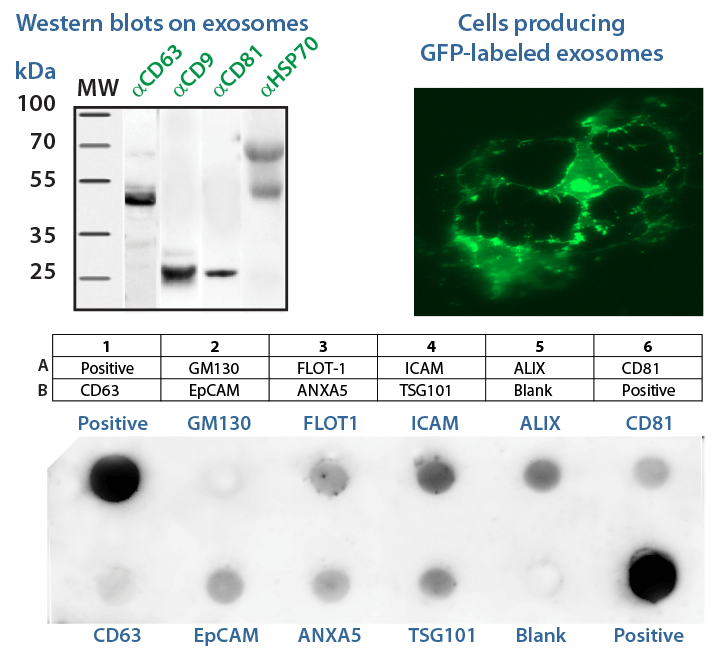
- Participants can analyze their own samples.
- Participants practice validated exosome workflows.
- Each participant receives a laboratory manual.
- Participants receive continuous education and support.
- Learn from experts with years of exosome experience.
- All instructors available for one-on-one discussions.
- Course takes place in sunny Palo Alto, CA.
Is this class for me?
Are you interested in studying exosomes?
Would you like to isolate, quantitate, and engineer exosomes?
Do you want to study exosomal RNA?
If you answered yes to any of the above, then this course is for you.
- In-depth exosome training course over 5 days with breakfast and lunch included (lodging and transportation not included).
- Ship your own samples to SBI for exosome analysis during the course (2 sample limit)!
Registration Information
TBD
- Academic participants: $1,500 per person (Cat# EC1000-A1)
- Corporate participants: $2,500 per person (Cat# EC1000-C1)
TBD
- Academic participants: $2,000 per person (Cat# EC1000-A2)
- Corporate participants: $3,000 per person (Cat# EC1000-C2)
Please send us an email with any questions you may have.
For more information or to submit your completed registration form, email us at tech@systembio.com
SBI Exosome Training Course - Sample Schedule
| MONDAY | TUESDAY | WEDNESDAY | THURSDAY | FRIDAY | |
|---|---|---|---|---|---|
| am Pre-meeting | Continental Breakfast | Continental Breakfast | Continental Breakfast | Continental Breakfast | Continental Breakfast |
| Morning 1 | Welcome, Orientation and Safety Lecture: Overview and background of Exosomes Exosome pre-course quiz and discussion |
Daily Review Lecture on that day’s experiments and tools used |
Daily Review Lecture on that day’s experiments and tools usedLecture: Exosome Biomarker Discovery |
Daily Review Lecture:Combining all exosome technologies and upcoming attractions |
Week Review Wet Lab: A) Analysis of exosome RNA qPCR results |
| Break 1 | Coffee/Tea | Coffee/Tea | Coffee/Tea | Coffee/Tea | Coffee/Tea |
| Wet Lab: A) Image cells producing XPack-GFP/XMIR-1 exosomes B) Isolation of exosomes from media using ExoQuick-TC and exosomes from serum samples |
Wet Lab: A) Harvesting of Exosomes, protein quantitation B) Load protein gels for westerns |
Wet Lab: Prepare and image Exo-Glow and Exo-Fect exosomes in cells B) Westerns – second day, add secondary antibody |
Wet Lab: A) Image XPack-GFP target cells B) Prepare target cell lysate RNA and XPack exosome RNA (SeraMir) C) Start cDNA synthesis |
Exosome post-course quiz and discussion Compile the week’s data on USB drives to take home |
|
| Lunch | Attendee introductions and exosome research interests | Guest Lecture: TBA | Special Lecture: Genome Editing with CRISPR/Cas9 | Guest Lecture: “Exosome-based Technology for the Development of Novel Therapies”, Dr. Alain Delcayre of ExoThera, Inc. | Distribution of Graduation Certificates |
| Afternoon 1 | Lecture: Introduction to engineering exosomes and experiments to label and transfect exosomes (XPack, XMIR, Exo-Glow, Exo-Fect, EV shuttles) | D) Quantify exosomes using EXOCET E) Western blots continued (transfer) |
C) Wash membranes D) Image Western blot results |
D) Set up qPCR plates for XMIR-1 and controls | |
| Break 2 | Coffee/Tea | Coffee/Tea | Coffee/Tea | Coffee/Tea | Coffee/Tea |
| Afternoon 2 | Wet Lab: Label exosome cargo with Exo-Glow, Exo-Fect exosomes with siRNA | F) Block membranes; add primary antibodies to Western blots G) Add XPack-GFP exosomes to target cellsH) Add Exo-Glow and Exo-Fected exosomes to target cells +/- EV- Entry |
Field trip to Google campus | Free time, Q&A discussions, data review | |
| 5:30pm class ends for the day | Dinner Event |

