Exo-Fect™ Exosome Transfection Kit
- Easy-to-use with a fast and straightforward loading protocol
- Introduces a wide range of biomolecules directly into isolated exosomes:
- RNAs, including siRNAs, miRNAs, and mRNAs
- DNAs, including plasmids
- Metabolites and other small molecules
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXFT10A-1 | Exo-Fect Exosome Transfection Kit | 10 Reactions | $347 |
|
||||
| EXFT20A-1 | Exo-Fect Exosome Transfection Kit | 20 Reactions | $642 |
|
||||
Overview
Overview
Putting exosomes to work: Load your cargo directly into isolated exosomes With Exo-Fect, you can turn isolated exosomes into cargo delivery vehicles that introduce RNAs, DNAs (including plasmids), and even small molecules into recipient cells. Simply combine isolated exosomes with Exo-Fect and your desired cargo, and in less than an hour your loaded exosomes will be ready to add to recipient cells.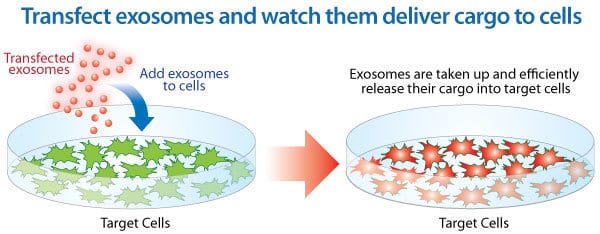
- Easy-to-use with a fast and straightforward loading protocol
- Introduces a wide range of biomolecules directly into isolated exosomes:
- RNAs, including siRNAs, miRNAs, and mRNAs
- DNAs, including plasmids
- Metabolites and other small molecules
- Enables non-viral transduction and stable cell line creation
- Provides an alternative gene delivery method for hard-to-transfect cells
References
How It Works
How It Works
Quickly and easily load cargo into isolated exosomes
Simply combine the isolated exosomes with Exo-Fect Reagent and cargo, perform two incubations for a total of 40 minutes, and then pellet your exosomes. The Kit comes with all of the reagents you need to load cargo into exosomes and concentrate them for delivery to target cells. The protocol takes less than an hour and is highly efficient at loading cargo into exosomes for transport and delivery.
Exo-Fect is also compatible with the EV-Entry Reagent, which maximizes cargo uptake by recipient cells.
Supporting Data
Supporting Data
See how Exo-Fect can be used to create cargo-delivering exosomes
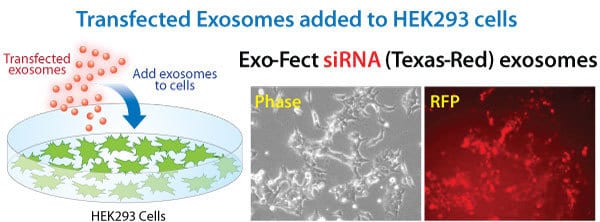
Exo-Fect efficiently loads small RNAs into exosomes for delivery to recipient cells
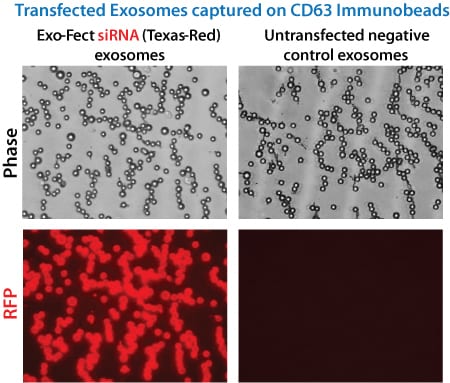
Figure 1. Exo-Fect efficiently loads siRNA into exosomes. Fluorescence imaging shows exosomes that were loaded with either a Texas Red end-labeled siRNA (left panels), or unlabeled siRNA (right panels) using Exo-Fect, and then immobilized onto Exo-Flow™ CD63 magnetic beads.
Figure 2. Exo-Fect transfected exosomes deliver siRNA cargo to recipient cells. The siRNA-loaded exosomes from Figure 1 were eluted from the Exo-Flow CD63 magnetic beads and then added to HEK293 cells. Fluorescence of the recipient cells indicates that the Texas Red-labeled siRNA cargo was successfully delivered.
Exo-Fect efficiently loads mRNAs and DNA plasmids into exosomes for delivery to recipient cells
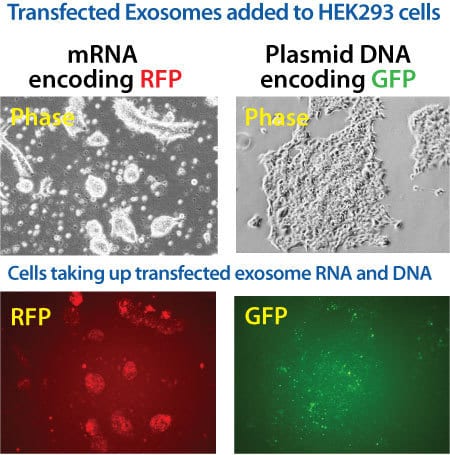
Figure 3. Exo-Fect loaded exosomes deliver mRNA and plasmid DNA into recipient cells. ExoFect Kits also work with larger nucleic acids, like mRNAs and plasmids. (Left panels) We used Exo-Fect to transfect 1 µg of an mRNA encoding RFP into exosomes. These exosomes were then added to HEK293 cells and imaged for RFP protein production after 24 hours. (Right panels) We used Exo-Fect to transfect 5 µg of plasmid DNA encoding a GFP gene into exosomes, and then added the plasmid-loaded exosomes to HEK293 cells. The cells were imaged for GFP protein presence after 48 hours. The appearance of RFP signal (mRNA cargo) and GFP signal (plasmid cargo) in recipient cells indicates successful cargo delivery.
Exo-Fect efficiently loads small molecules into exosomes for delivery to recipient cells
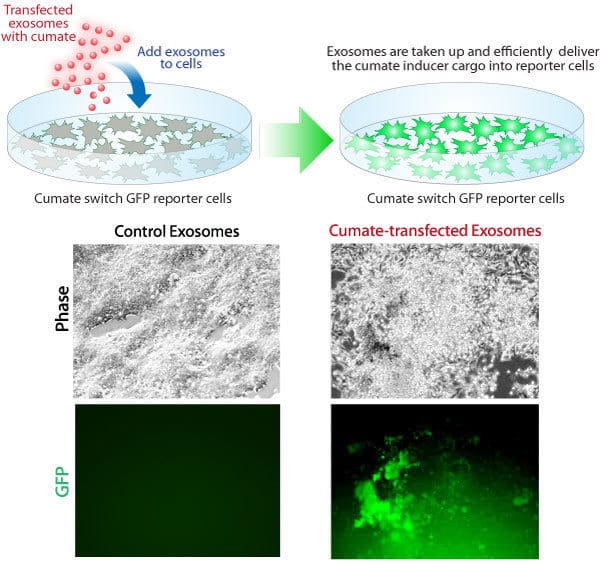
Figure 4. Exo-Fect can even be used to transfect small molecules into exosomes. We loaded cumate into exosomes using Exo-Fect, and then added loaded exosomes to cells containing a cumate-inducible GFP reporter construct. Only cumate-containing exosomes were able to induce GFP expression in recipient cells, indicating successful delivery of cumate.
FAQs
Documentation
Citations
Related Products
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXFT10A-1 | Exo-Fect Exosome Transfection Kit | 10 Reactions | $347 |
|
||||
| EXFT20A-1 | Exo-Fect Exosome Transfection Kit | 20 Reactions | $642 |
|
||||
Overview
Overview
Putting exosomes to work: Load your cargo directly into isolated exosomes With Exo-Fect, you can turn isolated exosomes into cargo delivery vehicles that introduce RNAs, DNAs (including plasmids), and even small molecules into recipient cells. Simply combine isolated exosomes with Exo-Fect and your desired cargo, and in less than an hour your loaded exosomes will be ready to add to recipient cells.
- Easy-to-use with a fast and straightforward loading protocol
- Introduces a wide range of biomolecules directly into isolated exosomes:
- RNAs, including siRNAs, miRNAs, and mRNAs
- DNAs, including plasmids
- Metabolites and other small molecules
- Enables non-viral transduction and stable cell line creation
- Provides an alternative gene delivery method for hard-to-transfect cells
References
How It Works
How It Works
Quickly and easily load cargo into isolated exosomes
Simply combine the isolated exosomes with Exo-Fect Reagent and cargo, perform two incubations for a total of 40 minutes, and then pellet your exosomes. The Kit comes with all of the reagents you need to load cargo into exosomes and concentrate them for delivery to target cells. The protocol takes less than an hour and is highly efficient at loading cargo into exosomes for transport and delivery.
Exo-Fect is also compatible with the EV-Entry Reagent, which maximizes cargo uptake by recipient cells.
Supporting Data
Supporting Data
See how Exo-Fect can be used to create cargo-delivering exosomes
Exo-Fect efficiently loads small RNAs into exosomes for delivery to recipient cells

Figure 1. Exo-Fect efficiently loads siRNA into exosomes. Fluorescence imaging shows exosomes that were loaded with either a Texas Red end-labeled siRNA (left panels), or unlabeled siRNA (right panels) using Exo-Fect, and then immobilized onto Exo-Flow™ CD63 magnetic beads.
Figure 2. Exo-Fect transfected exosomes deliver siRNA cargo to recipient cells. The siRNA-loaded exosomes from Figure 1 were eluted from the Exo-Flow CD63 magnetic beads and then added to HEK293 cells. Fluorescence of the recipient cells indicates that the Texas Red-labeled siRNA cargo was successfully delivered.
Exo-Fect efficiently loads mRNAs and DNA plasmids into exosomes for delivery to recipient cells

Figure 3. Exo-Fect loaded exosomes deliver mRNA and plasmid DNA into recipient cells. ExoFect Kits also work with larger nucleic acids, like mRNAs and plasmids. (Left panels) We used Exo-Fect to transfect 1 µg of an mRNA encoding RFP into exosomes. These exosomes were then added to HEK293 cells and imaged for RFP protein production after 24 hours. (Right panels) We used Exo-Fect to transfect 5 µg of plasmid DNA encoding a GFP gene into exosomes, and then added the plasmid-loaded exosomes to HEK293 cells. The cells were imaged for GFP protein presence after 48 hours. The appearance of RFP signal (mRNA cargo) and GFP signal (plasmid cargo) in recipient cells indicates successful cargo delivery.
Exo-Fect efficiently loads small molecules into exosomes for delivery to recipient cells

Figure 4. Exo-Fect can even be used to transfect small molecules into exosomes. We loaded cumate into exosomes using Exo-Fect, and then added loaded exosomes to cells containing a cumate-inducible GFP reporter construct. Only cumate-containing exosomes were able to induce GFP expression in recipient cells, indicating successful delivery of cumate.