96-well ExoELISA Plate
Products
Overview
Overview
Additional plates optimized for our ExoELISA Kits For researchers who just need additional ExoELISA plates, we offer 96-well plates as a pack of ten. Each 96-well plate comes as a 12 strips of 8 wells each to maximize your flexibility—unused strips can be stored at room temperature for later use. Choose the exosome quantitation method that’s best for your studies| ExoELISA-ULTRA Complete Kits | EXOCET | FluoroCet | |
|---|---|---|---|
| Use | For fast and sensitive antibody-based quantitation of exosomes | For fast quantitation of extracellular vesicles with moderate sample input requirements | For the most sensitive quantitation of extracellular vesicles with very low sample input requirements |
| Detection method | Antibody | Enzymatic | Enzymatic |
| Quantitation chemistry | Enzymatic (HRP) | Colorimetric | Fluorescent |
| Total protocol time | 4 hours (no overnight incubation) | 20 min | 60 min |
| Input sample amount (protein equivalent) | 1 – 200 µg | 50 µg | <1 µg |
| Learn More | ExoELISA-ULTRA CD63 ExoELISA-ULTRA CD81 ExoELISA-ULTRA CD9 | EXOCET | FluoroCet |
How It Works
How It Works
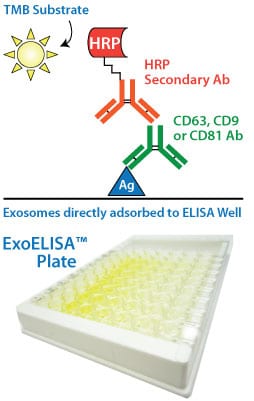
Our ExoELISA Kits have all the reagents you need to run the ELISA—just add lysed exosome particles. The kits are compatible with exosomes isolated using most methods, including ExoQuick®, ExoQuick-TC®, or ultracentrifugation.
The lysed exosome particles (and, thus, exosomal proteins) are directly immobilized onto the wells of the microtiter plate, and after binding, a blocking agent is added to prevent non-specific binding of the primary detection antibody (anti-CD9, -CD63, or -CD81). Following addition of the primary antibody, a secondary antibody (goat anti-rabbit) linked to horseradish peroxidase (HRP) is also added to amplify the signal and increase assay sensitivity.
The amount of the exosome marker (CD9, CD63, or CD81) is measured via activity of the bound HRP-secondary antibody using a colorimetric assay with extra-sensitive TMB as the substrate. The accumulation of colored product is proportional to the amount of marker present in each well, and is measured using a microtiter plate reader at 450 nm absorbance.
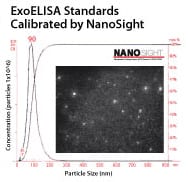
Each ExoELISA Kit includes a set of standards calibrated to a known amount of exosome particles as determined by NanoSight analysis. These standards can be used to generate a calibration curve enabling quantitation of exosomes carrying the marker of interest from the ExoELISA data.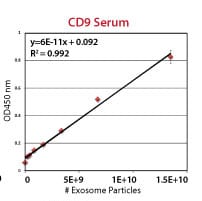
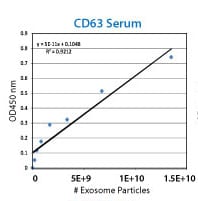
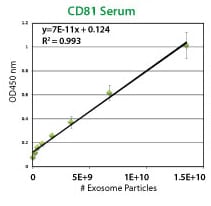
Supporting Data
FAQs
Resources
Related Products
Citations
-
Han, D, et al. (2024) Current Technology for Production, Isolation, and Quality Control of Extracellular Vesicles. Biomedical Applications of Extracellular Vesicles. 2024;:117-146. Link: Biomedical Applications of Extracellular Vesicles
-
Bhagwan Valjee, R, et al. (2024) Investigation of exosomal tetraspanin profile in sepsis patients as a promising diagnostic biomarker. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2024;:1-12. PM ID: 38354024
-
Pallares-Rusiñol, A, et al. (2023) Advances in exosome analysis. Advances in clinical chemistry. 2023; 112:69-117. PM ID: 36642486
-
Lee, S, et al. (2023) Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification. Experimental & molecular medicine. 2023;. PM ID: 36964252
-
Cai, J, et al. (2023) Exosomes Derived From Kartogenin-Preconditioned Mesenchymal Stem Cells Promote Cartilage Formation and Collagen Maturation for Enthesis Regeneration in a Rat Model of Chronic Rotator Cuff Tear. The American journal of sports medicine. 2023; 51(5):1267-1276. PM ID: 36917828
-
Nguyen, CM, et al. (2023) Placental Exosomes as Biomarkers for Maternal Diseases: Current Advances in Isolation, Characterization, and Detection. ACS sensors. 2023; 8(7):2493-2513. PM ID: 37449399
-
Taha, H. (2023) Biomarkers in CNS-originating Extracellular Vesicles for Parkinson’s disease and Multiple System Atrophy. Thesis. 2023;. Link: Thesis
-
Ayala-Mar, S & Gonzalez-Valdez, J. (2023) Research and Development of Emerging Technologies for Exosome-based Cancer Diagnostics and Therapeutics. laccei.org. 2023;. Link: laccei.org
-
Rowart, P, et al. (2023) Fast and Efficient Isolation of Exosomes from Stem Cells Using a Combination of Single-Use Bioreactors, High-Speed-and Ultracentrifugation. eppendorf.com. 2023;. Link: eppendorf.com
-
Gandham, SK, Attarwala, HZ & Amiji, MM. (2022) Mathematical Modeling and Experimental Validation of Extracellular Vesicle-Mediated Tumor Suppressor MicroRNA Delivery and Propagation in Ovarian Cancer Cells. Molecular pharmaceutics. 2022;. PM ID: 36226722
-
Bazoer, J. (2022) Regulatory T Cell Derived EVs-Designing Novel Immune Based Therapies to Prolong Lifespan of Transplanted Tissue. Thesis. 2022;. Link: Thesis
-
Ntsethe, A & Mackraj, I. (2022) An Investigation of Exosome Concentration and Exosomal microRNA (miR-155 and miR-222) Expression in Pregnant Women with Gestational Hypertension and Preeclampsia. International journal of women's health. 2022; 14:1681-1689. PM ID: 36514348
-
Turner, A, et al. (2021) Donor specific phenotypic variation in hiPSC cardiomyocyte-derived exosomes impacts endothelial cell function. American journal of physiology. Heart and circulatory physiology. 2021;. PM ID: 33416449
-
Salem, ZA, Kamel, AHM & AbuBakr, N. (2021) Salivary exosomes as a new therapy to ameliorate diabetes mellitus and combat xerostomia and submandibular salivary glands dysfunction in diabetic rats. Journal of molecular histology. 2021;. PM ID: 33389429
-
Born, LJ, et al. (2021) HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Advanced healthcare materials. 2021;:e2002070. PM ID: 33870645
-
Chen, Y, et al. (2020) Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020; 10(3):1454-1478. PM ID: 31938074
-
Wernly, B, et al. (2020) Anti-CD3 Antibody Treatment Reduces Scar Formation in a Rat Model of Myocardial Infarction. Cells. 2020; 9(2). PM ID: 31991811
-
Hernández-Walias, F, et al. (2020) New signatures of poor CD4 cell recovery after suppressive antiretroviral therapy in HIV-1-infected individuals: involvement of miR-192, IL-6, sCD14 and miR-144. Sci Rep. 2020; 10(1):2937. PM ID: 32076107
-
Maduray, K, Moodley, J & Mackraj, I. (2020) The impact of circulating exosomes derived from early and late onset pre-eclamptic pregnancies on inflammatory cytokine secretion by BeWo cells. Eur. J. Obstet. Gynecol. Reprod. Biol.. 2020; 247:156-162. Link: Eur. J. Obstet. Gynecol. Reprod. Biol.
-
Hernández-Walias, FJ, et al. (2020) Risk, Diagnostic and Predictor Factors for Classical Hodgkin Lymphoma in HIV-1-Infected Individuals: Role of Plasma Exosome-Derived miR-20a and miR-21. J Clin Med. 2020; 9(3). PM ID: 32168859
- See More
Products
Overview
Overview
Additional plates optimized for our ExoELISA Kits For researchers who just need additional ExoELISA plates, we offer 96-well plates as a pack of ten. Each 96-well plate comes as a 12 strips of 8 wells each to maximize your flexibility—unused strips can be stored at room temperature for later use. Choose the exosome quantitation method that’s best for your studies| ExoELISA-ULTRA Complete Kits | EXOCET | FluoroCet | |
|---|---|---|---|
| Use | For fast and sensitive antibody-based quantitation of exosomes | For fast quantitation of extracellular vesicles with moderate sample input requirements | For the most sensitive quantitation of extracellular vesicles with very low sample input requirements |
| Detection method | Antibody | Enzymatic | Enzymatic |
| Quantitation chemistry | Enzymatic (HRP) | Colorimetric | Fluorescent |
| Total protocol time | 4 hours (no overnight incubation) | 20 min | 60 min |
| Input sample amount (protein equivalent) | 1 – 200 µg | 50 µg | <1 µg |
| Learn More | ExoELISA-ULTRA CD63 ExoELISA-ULTRA CD81 ExoELISA-ULTRA CD9 | EXOCET | FluoroCet |
How It Works
How It Works
Our ExoELISA Kits have all the reagents you need to run the ELISA—just add lysed exosome particles. The kits are compatible with exosomes isolated using most methods, including ExoQuick®, ExoQuick-TC®, or ultracentrifugation.
The lysed exosome particles (and, thus, exosomal proteins) are directly immobilized onto the wells of the microtiter plate, and after binding, a blocking agent is added to prevent non-specific binding of the primary detection antibody (anti-CD9, -CD63, or -CD81). Following addition of the primary antibody, a secondary antibody (goat anti-rabbit) linked to horseradish peroxidase (HRP) is also added to amplify the signal and increase assay sensitivity.
The amount of the exosome marker (CD9, CD63, or CD81) is measured via activity of the bound HRP-secondary antibody using a colorimetric assay with extra-sensitive TMB as the substrate. The accumulation of colored product is proportional to the amount of marker present in each well, and is measured using a microtiter plate reader at 450 nm absorbance.
Each ExoELISA Kit includes a set of standards calibrated to a known amount of exosome particles as determined by NanoSight analysis. These standards can be used to generate a calibration curve enabling quantitation of exosomes carrying the marker of interest from the ExoELISA data.



Supporting Data
FAQs
Citations
-
Han, D, et al. (2024) Current Technology for Production, Isolation, and Quality Control of Extracellular Vesicles. Biomedical Applications of Extracellular Vesicles. 2024;:117-146. Link: Biomedical Applications of Extracellular Vesicles
-
Bhagwan Valjee, R, et al. (2024) Investigation of exosomal tetraspanin profile in sepsis patients as a promising diagnostic biomarker. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2024;:1-12. PM ID: 38354024
-
Pallares-Rusiñol, A, et al. (2023) Advances in exosome analysis. Advances in clinical chemistry. 2023; 112:69-117. PM ID: 36642486
-
Lee, S, et al. (2023) Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification. Experimental & molecular medicine. 2023;. PM ID: 36964252
-
Cai, J, et al. (2023) Exosomes Derived From Kartogenin-Preconditioned Mesenchymal Stem Cells Promote Cartilage Formation and Collagen Maturation for Enthesis Regeneration in a Rat Model of Chronic Rotator Cuff Tear. The American journal of sports medicine. 2023; 51(5):1267-1276. PM ID: 36917828
-
Nguyen, CM, et al. (2023) Placental Exosomes as Biomarkers for Maternal Diseases: Current Advances in Isolation, Characterization, and Detection. ACS sensors. 2023; 8(7):2493-2513. PM ID: 37449399
-
Taha, H. (2023) Biomarkers in CNS-originating Extracellular Vesicles for Parkinson’s disease and Multiple System Atrophy. Thesis. 2023;. Link: Thesis
-
Ayala-Mar, S & Gonzalez-Valdez, J. (2023) Research and Development of Emerging Technologies for Exosome-based Cancer Diagnostics and Therapeutics. laccei.org. 2023;. Link: laccei.org
-
Rowart, P, et al. (2023) Fast and Efficient Isolation of Exosomes from Stem Cells Using a Combination of Single-Use Bioreactors, High-Speed-and Ultracentrifugation. eppendorf.com. 2023;. Link: eppendorf.com
-
Gandham, SK, Attarwala, HZ & Amiji, MM. (2022) Mathematical Modeling and Experimental Validation of Extracellular Vesicle-Mediated Tumor Suppressor MicroRNA Delivery and Propagation in Ovarian Cancer Cells. Molecular pharmaceutics. 2022;. PM ID: 36226722
-
Bazoer, J. (2022) Regulatory T Cell Derived EVs-Designing Novel Immune Based Therapies to Prolong Lifespan of Transplanted Tissue. Thesis. 2022;. Link: Thesis
-
Ntsethe, A & Mackraj, I. (2022) An Investigation of Exosome Concentration and Exosomal microRNA (miR-155 and miR-222) Expression in Pregnant Women with Gestational Hypertension and Preeclampsia. International journal of women's health. 2022; 14:1681-1689. PM ID: 36514348
-
Turner, A, et al. (2021) Donor specific phenotypic variation in hiPSC cardiomyocyte-derived exosomes impacts endothelial cell function. American journal of physiology. Heart and circulatory physiology. 2021;. PM ID: 33416449
-
Salem, ZA, Kamel, AHM & AbuBakr, N. (2021) Salivary exosomes as a new therapy to ameliorate diabetes mellitus and combat xerostomia and submandibular salivary glands dysfunction in diabetic rats. Journal of molecular histology. 2021;. PM ID: 33389429
-
Born, LJ, et al. (2021) HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Advanced healthcare materials. 2021;:e2002070. PM ID: 33870645
-
Chen, Y, et al. (2020) Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020; 10(3):1454-1478. PM ID: 31938074
-
Wernly, B, et al. (2020) Anti-CD3 Antibody Treatment Reduces Scar Formation in a Rat Model of Myocardial Infarction. Cells. 2020; 9(2). PM ID: 31991811
-
Hernández-Walias, F, et al. (2020) New signatures of poor CD4 cell recovery after suppressive antiretroviral therapy in HIV-1-infected individuals: involvement of miR-192, IL-6, sCD14 and miR-144. Sci Rep. 2020; 10(1):2937. PM ID: 32076107
-
Maduray, K, Moodley, J & Mackraj, I. (2020) The impact of circulating exosomes derived from early and late onset pre-eclamptic pregnancies on inflammatory cytokine secretion by BeWo cells. Eur. J. Obstet. Gynecol. Reprod. Biol.. 2020; 247:156-162. Link: Eur. J. Obstet. Gynecol. Reprod. Biol.
-
Hernández-Walias, FJ, et al. (2020) Risk, Diagnostic and Predictor Factors for Classical Hodgkin Lymphoma in HIV-1-Infected Individuals: Role of Plasma Exosome-Derived miR-20a and miR-21. J Clin Med. 2020; 9(3). PM ID: 32168859
- See More